Nitroacetic acid
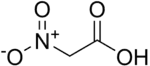
Nitroacetic acid is the chemical compound with the formula (NO2)CH2CO2H. This substituted carboxylic acid is used as a potential precursor to nitromethane, commonly used as a fuel in drag racing and as an organic reagent in chemical synthesis.
 | |
| Names | |
|---|---|
| IUPAC name
2-Nitroacetic acid | |
| Other names
Nitro acetate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.249.741 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2H3NO4 | |
| Molar mass | 105.049 g·mol−1 |
| Density | 1.5±0.1 g/cm3 |
| Acidity (pKa) | 1.68 [1] |
| Hazards | |
| Flash point | 150.6±11.1 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
Nitroacetic acid can be synthesized by adding cold chloroacetic acid into a cold, slightly alkaline aqueous solution, followed by mixing with aqueous sodium nitrite solution. It is important during this procedure not to make the solution too alkaline and to keep it cold to prevent the formation of sodium glycolate.
Reactions
Nitroacetic acid can be used in the production of nitromethane by thermal decarboxylation of a corresponding salt to at 80 °C.[2]
References
- Dippy, J. F. J.; Hughes, S. R. C.; Rozanski, A. (1959). "498. The dissociation constants of some symmetrically disubstituted succinic acids". Journal of the Chemical Society (Resumed): 2492. doi:10.1039/jr9590002492.
- F. C. Whitmore and Marion G. Whitmore (1923). "Nitromethane". Organic Syntheses. 3: 83.; Collective Volume, 1, p. 401
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.