Naphthylvinylpyridine
Naphthylvinylpyridine (NVP) is a naphthalene derivative that possesses anticholinergic activity similar to that of atropine. However, NVP's method of acetylcholine (ACh) antagonism involves inhibiting the enzyme choline acetyltransferase.[1]
 | |
| Names | |
|---|---|
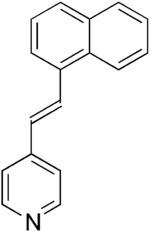
| IUPAC name
4-[(E)-2-naphthalen-1-ylethenyl]pyridine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.203.347 |
| MeSH | D009286 |
PubChem CID |
|
| |
| |
| Properties | |
| C17H13N | |
| Molar mass | 231.29 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Several NVP derivatives have been synthesized and evaluated for their ability to inhibit choline acetyltransferase and protect against nerve toxins.[2][3]
References
- Haubrich, DR; Goldberg, ME (1975). "Homovanillic acid concentration in the rat brain: Effect of a choline acetyltransferase inhibitor and comparison with cholinergic and dopaminergic agents". Neuropharmacology. Squibb Institute for Medical Research. 14 (3): 211–214. doi:10.1016/0028-3908(75)90007-6. PMID 1134625. S2CID 28216329.
- Cozzari, Costantino; Hartman, BK (1983). "Synthesis of a naphthylvinylpyridine derivative and its use for affinity chromatography of choline acetyltransferase". Analytical Biochemistry. Department of Psychiatry and Neurobiology, Washington University School of Medicine. 133 (1): 120–125. doi:10.1016/0003-2697(83)90231-2. PMID 6638474.
- Gray, AP; Henderson, TR (1988). "Approaches to protection against nerve agent poisoning". J Med Chem. Dynamac Corporation. 31 (4): 807–814. doi:10.1021/jm00399a022. PMID 3351860.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.