Naphthionic acid
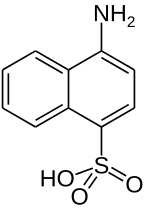
Naphthionic acid is an organic compound with the formula C10H6(SO3H)(NH2). It is one of several aminonaphthalenesulfonic acids, derivatives of naphthalene containing both amine and sulfonic acid functional groups. It is a white solid, although commercial samples can appear gray.[1] It is used in the synthesis of azo dyes such as Rocceline (a. k. a. Solid Red A), during which the amino group of the acid (in the form of a salt) is diazotated and then coupled with, in the case mentioned, β-naphthol. It is prepared by treating 1-aminonaphthalene with sulfuric acid.[2]
 | |
| Names | |
|---|---|
| IUPAC name
4-aminonaphthalene-1-sulfonic acid | |
| Other names
Piria's acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.001.425 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H9NO3S | |
| Molar mass | 223.24 |
| Appearance | white solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- 4-Amino-1-naphthalenesulfonic acid; MSDS No. 250619; Sigma–Aldrich Chemie GmbH: Steinheim, 29 Dec 2011.
- Gerald Booth "Naphthalene Derivatives" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a17_009.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.