Myrophine
Myrophine (Myristylbenzylmorphine) is an opiate analogue that was developed in 1952. It is a derivative of morphine.
 | |
| Clinical data | |
|---|---|
| Other names | Myristylbenzylmorphine |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
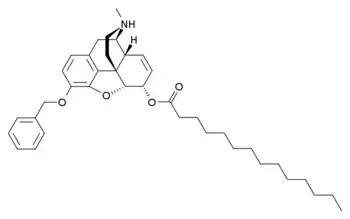
| Formula | C38H51NO4 |
| Molar mass | 585.829 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Myrophine is substituted with a 3-benzyl group and a 6-myristyl chain. It is metabolised to form benzylmorphine and then further to morphine, and so is a long-acting prodrug for morphine, but with a slow onset of effects. It is weaker than morphine as an analgesic but longer-lasting in effects, and was thought to have more local anesthetic effect than morphine, though with a somewhat greater tendency to cause histamine reactions like itching and rash. In addiction studies conducted in human subjects in the 1950s, myrophine did not substitute for morphine in withdrawal, did not produce notable morphine-like effects, and did not produce addiction or dependence regardless of dose or how it was administered. Consequently, it was thought to be useful in treating pain in addicts who were being detoxified from other opioid drugs.[1]
It is a Schedule I drug in the US, considered to have high potential for abuse and no medical applications, and is controlled under international drug conventions. Myrophine is almost invariably used as the hydrochloride (free base conversion ratio 0.94) and has a DEA Administrative Controlled Substance Control Number of 9308. Myrophine is a Class A controlled substance in the UK,[2] and is on the UN's Yellow list.
References
- Eddy NB, Halbach H, Braenden OJ (1957). "Synthetic substances with morphine-like effect: clinical experience; potency, side-effects, addiction liability" (PDF). Bulletin of the World Health Organization. 17 (4–5): 569–863. PMC 2537678. PMID 13511135.
- "Part I Class A Drugs - IsomerDesign". 2012-03-03. Retrieved 2012-03-24.