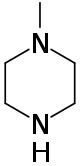
Methylpiperazine
N-Methylpiperazine is a heterocyclic organic compound.
 | |
| Names | |
|---|---|
| IUPAC name
N-Methylpiperazine | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.309 |
| EC Number |
|
PubChem CID |
|
| UNII | |
| UN number | 2920 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H12N2 | |
| Molar mass | 100.165 g·mol−1 |
| Melting point | −6 °C (21 °F; 267 K)[1] |
| Boiling point | 138 °C (280 °F; 411 K)[1] |
| Hazards | |
| Safety data sheet | FischerSci |
| GHS pictograms |     |
| GHS Signal word | Danger |
| H226, H312, H314, H317, H318, H330, H331, H332 | |
| P210, P233, P240, P241, P242, P243, P260, P261, P264, P271, P272, P280, P284, P301+330+331, P302+352, P303+361+353, P304+312, P304+340, P305+351+338, P310, P311, P312, P320, P321, P322 | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Related compounds |
Piperazine, 4-methylpyridine |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Uses
N-Methylpiperazine is a common building block used in organic synthesis.[2] For example, N-methylpiperazine is used in the manufacture of various pharmaceutical drugs including cyclizine,[3] meclizine, and sildenafil.
The lithium salt, lithium N-methylpiperazide, is used as a reagent in organic synthesis for protection of aryl aldehydes.[4]
Synthesis
Industrially, N-methylpiperazine is produced by reacting diethanolamine and methylamine at 250 bar and 200 °C.[5][6]
References
- "1-Methylpiperazine". Chemistry Dashboard. Environmental Protection Agency.
- "1-methylpiperazine". European Chemicals Agency.
- Vardanyan, Ṛuben & Hruby, Victor J. Synthesis of Essential Drugs. p. 226.CS1 maint: multiple names: authors list (link)
- Comins, Daniel L.; Joseph, Sajan P. (2001). "Lithium N-Methylpiperazide". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rl128. ISBN 0471936235.
- US 4845218, "Preparation of N-Methylpiperazine", issued 1989-07-04
- "Catalytic synthesis of N-methylpiperazine from diethanolamine and methylamine by cyclodehydration reaction" (PDF). Indian Journal of Chemical Technology. 1 (November): 359–360. 1994.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
