Methylenetriphenylphosphorane
Methylenetriphenylphosphorane is an organophosphorus compound with the formula Ph3PCH2. It is the parent member of the phosphorus ylides, popularly known as Wittig reagents. It is a highly polar, highly basic species.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Methylene(triphenyl)phosphorane | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C19H17P | |
| Appearance | yellow solid |
| Density | 1.19 g/cm3 |
| decompose | |
| Solubility | THF |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation and use
Methylenetriphenylphosphorane is prepared from methyltriphenylphosphonium bromide by its deprotonation using a strong base like butyllithium:[1]
- Ph3PCH3Br + BuLi → Ph3PCH2 + LiBr + BuH
The phosphorane is generally not isolated, instead it is used in situ. The estimated pKa of this carbon acid is near 15.[2]
Methylenetriphenylphosphorane is used to replace oxygen centres in aldehydes and ketones with a methylene group:
- R2CO + Ph3PCH2 → R2C=CH2 + Ph3PO
The phosphorus-containing product is triphenylphosphine oxide.
Structure
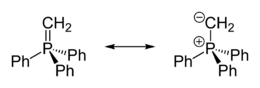
Crystallographic characterization of the colourless ylide reveals that the phosphorus atom is approximately tetrahedral. The PCH2 centre is planar and the P=CH2 distance is 1.661 Å, which is much shorter than the P-Ph distances (1.823 Å).[3] The compound is usually described as a combination of two resonance structures:
- Ph3P+CH2− ↔ Ph3P=CH2
2.png.webp)
Related compounds
Electron-withdrawing groups (EWGs) enhance the ease of deprotonation of phosphonium salts. This behavior is illustrated by triphenylcarbethoxymethylphosphonium derived from chloroacetic acid esters and triphenylphosphine. It deprotonation requires only sodium hydroxide. The resulting triphenylcarbethoxymethylenephosphorane is somewhat air-stable. It is however less reactive than ylides lacking EWGs. For example they usually fail to react with ketones, necessitating the use of the Horner–Wadsworth–Emmons reaction as an alternative. Such stabilized ylides usually give rise to an E-alkene product when they react, rather than the more usual Z-alkene.

Although these ylides are "electron-rich", they are susceptible to deprotonation. Treatment of Me3PCH2 with butyl lithium affords Me2P(CH2)2Li.[5]
Having carbanion-like properties, lithiated ylides function as ligands. Thus Me2P(CH2)2Li is a bidentate ligand.[6][5]
Related reagents
References
- Wittig, Georg; Schoellkopf, U. (1960). "Methylenecyclohexane". 40: 66. doi:10.15227/orgsyn.040.0066. Cite journal requires
|journal=(help) - Ling-Chung, Sim; Sales, Keith D.; Utley, James H. P. (1990). "Measurement of pKa Values for Phosphonium Salts via the Kinetics of Proton Transfer to an Electrogenerated Base". Journal of the Chemical Society, Chemical Communications (9): 662. doi:10.1039/C39900000662.
- Bart, J. C. J. "Structure of the non-stabilized phosphonium ylid methylenetriphenylphosphorane". Journal of the Chemical Society B. 1969: 350–365. doi:10.1039/J29690000350.
- Tonner, Ralf; Oexler, Florian; Neumueller, Bernhard; Petz, Wolfgang; Frenking, Gernot (2006). "Carbodiphosphoranes: The Chemistry of Divalent Carbon(0)". Angewandte Chemie International Edition. 45 (47): 8038–8042. doi:10.1002/anie.200602552. PMID 17075933.CS1 maint: uses authors parameter (link)
- Fackler, J. P.; Basil, J. D. (1982). "Oxidative Addition of Methyl Iodide to a Dinuclear gold(I) Complex. The X-Ray Crystal Structure of Bis[mu-(Dimethyldimethylenephosphoranyl-C,C)]-iodomethyldigold(II)(Au-Au), Au2[(CH2)2P(CH3)2]2(CH3)I". Organometallics. 1 (6): 871–873. doi:10.1021/om00066a021.CS1 maint: uses authors parameter (link)
- Schmidbaur, H. (1983). "Phosphorus Ylides in the Coordination Sphere of Transition Metals: An Inventory". Angewandte Chemie International Edition in English. 22 (12): 907–927. doi:10.1002/anie.198309071.