Mesitol
Mesitol (2,4,6-trimethylphenol) is an aromatic chemical compound having three methyl groups and one hydroxy group. The name and structure of mesitol derives from the combination of mesitylene and phenol.
 | |
| Names | |
|---|---|
| IUPAC name
2,4,6-Trimethylphenol | |
| Other names
Hydroxymesitylene; Mesityl alcohol | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.007.655 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H12O | |
| Molar mass | 136.194 g·mol−1 |
| Melting point | 70–72 °C (158–162 °F; 343–345 K)[1] |
| Boiling point | 220 °C (428 °F; 493 K)[1] |
| 1.01 g/l | |
| Hazards | |
| GHS pictograms | 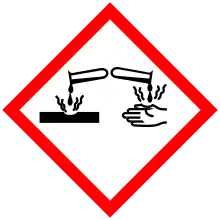 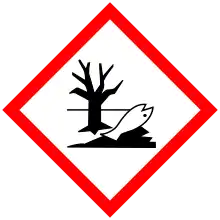 |
| GHS Signal word | Danger |
| H314, H318, H411 | |
| P260, P264, P273, P280, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P321, P363, P391, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
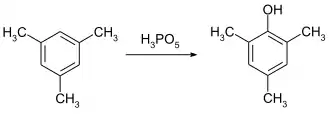
Mesitol can be obtained by reaction of mesitylene with peroxymonophosphoric acid:[2]
References
- "2,4,6-Trimethylphenol". Sigma-Aldrich.
- Ogata, Yoshiro; Sawaki, Yasuhiko; Tomizawa, Kohtaro; Ohno, Takashi (1981). "Aromatic hydroxylation with peroxymonophosphoric acid". Tetrahedron. 37 (8): 1485. doi:10.1016/S0040-4020(01)92087-3.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.