List of benzimidazole opioids
A number of benzimidazole opioids have been developed, with the best known being the highly potent but never marketed etonitazene. The structure-activity relationship has been explored to a reasonable extent. The optimal substitution pattern is fairly tightly defined (i.e. N,N-diethyl on the amine nitrogen, 4-ethoxy on the benzyl ring and 5-nitro on the benzimidazole ring), but even derivatives incorporating only some of these features are still potent opioids. If a methyl or carboxamide group is added on the alpha carbon of the benzyl group, or the benzyl is replaced by 2-phenylethyl, compounds of similar activity are obtained. Relative analgesic activity values are derived from tests on mice and cannot be extrapolated directly to humans, though the same general activity trends apply.[1][2][3][4][5][6][7][8][9][10][11][12][13]
A 2019 publication[14] has shown the possibility the previously assumed binding position of the benzimidazole class,[15] acting as a semi-rigid fentanyl analogue may be incorrect. Based on a large scale analysis of known opioid receptor ligands a template was created through manual overlaying and alignment which has identified several mu-specific areas within the receptor. In this analysis it is noted etonitazene now more closely matches another separate mu-specific region sharing very only a small area in common with the fentanyl class.
Table of benzimidazole opioids
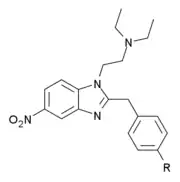
| Chemical structure | Drug name | Ring substitution | Analgesic potency (morphine = 1) | PubChem | CAS number |
|---|---|---|---|---|---|
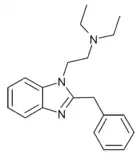 |
1-diethylaminoethyl-2-benzyl-benzimidazole | hydrogen | 0.1 | 28787 | |
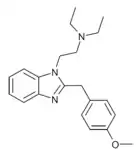 |
Metodesnitazene (Metazene) | 4-methoxy | 1 | 26412 | 14030-77-4 1071546-40-1 (HCl) |
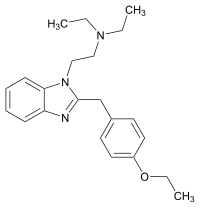 |
Etodesnitazene (Etazene) | 4-ethoxy | 70 | 149797386 | |
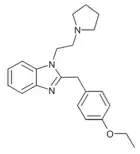 |
Etodesnitazene pyrrolidine analogue | 4-ethoxy | 20 | ||
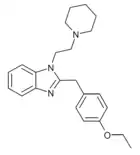 |
Etodesnitazene piperidine analogue | 4-ethoxy | 10 | ||
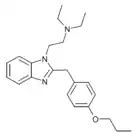 |
Protodesnitazene | 4-(n-propoxy) | 10 | ||
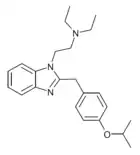 |
Isotodesnitazene | 4-isopropoxy | ~75 | ||
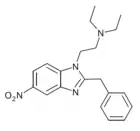 |
Nitazene | hydrogen | 2 | 15327524 | |
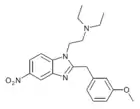 |
meta-Metonitazene | 3-methoxy | 2 | ||
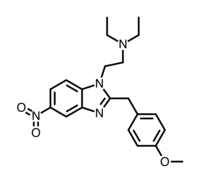 |
Metonitazene | 4-methoxy | 100 | 53316366 | 14680-51-4 |
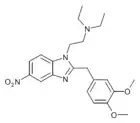 |
Dimetonitazene | 3,4-dimethoxy | 10 | ||
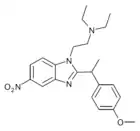 |
α-methyl-metonitazene | 4-methoxy | 50 | ||
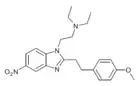 |
Metonitazene phenethyl homologue | 4-methoxy | 50 | ||
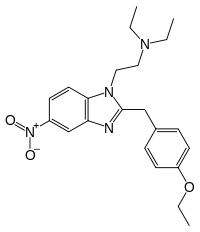 |
Etonitazene | 4-ethoxy | 1000 | 13493 | 911-65-9 |
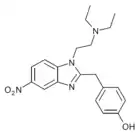 |
O-Desmethyl-etonitazene | 4-hydroxy | 1 | 94758-81-3 | |
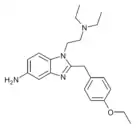 |
Etonitazene 5-amino metabolite | 4-ethoxy | 2 | 13408927 | |
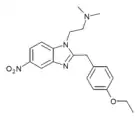 |
Etonitazene N,N-dimethyl analogue | 4-ethoxy | 20 | ||
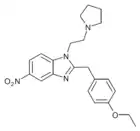 |
Etonitazepyne | 4-ethoxy | |||
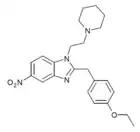 |
Etonitazene piperidine analogue | 4-ethoxy | 100 | ||
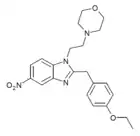 |
Etonitazene morpholine analogue | 4-ethoxy | 2 | ||
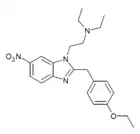 |
Etonitazene 6-nitro isomer | 4-ethoxy | 20 | ||
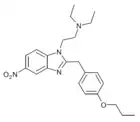 |
Protonitazene | 4-(n-propoxy) | 200 | 119276-01-6 | |
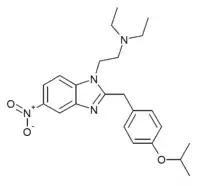 |
Isotonitazene | 4-isopropoxy | 500 | 145721979 | 14188-81-9 |
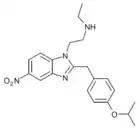 |
N-desethyl-isotonitazene | 4-isopropoxy | ~1000 | ||
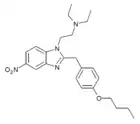 |
Butonitazene | 4-butoxy | 5 | 95810-54-1 | |
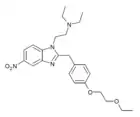 |
Etoetonitazene | 4-ethoxyethoxy | 50 | ||
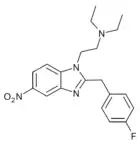 |
Fluonitazene | 4-fluoro | 1 | 2728-91-8 | |
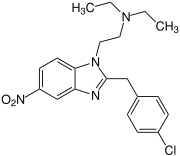 |
Clonitazene | 4-chloro | 3 | 62528 | 3861-76-5 |
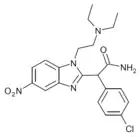 |
α-carboxamido-clonitazene | 4-chloro | 3 | ||
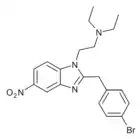 |
Bronitazene | 4-bromo | 5 | ||
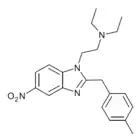 |
Methylnitazene | 4-methyl | 10 | ||
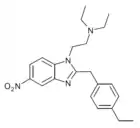 |
Ethylnitazene | 4-ethyl | 20 | ||
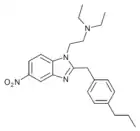 |
Propylnitazene | 4-propyl | 50 | ||
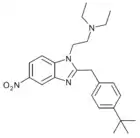 |
t-Butylnitazene | 4-(tert-butyl) | 2 | ||
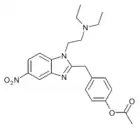 |
Acetoxynitazene | 4-acetoxy | 5 | ||
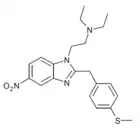 |
Methylthionitazene | 4-methylthio | 50 | ||
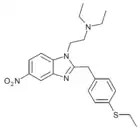 |
Ethylthionitazene | 4-ethylthio | 30 | ||
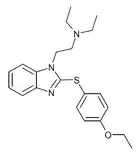 |
Etodesnitazene phenylthiol analogue | 4-ethoxy | 1 | 21045 | |
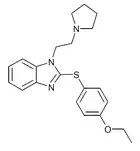 |
Etodesnitazene phenylthiol / pyrrolidine analogue | 4-ethoxy | 2 | 19846499 | |
See also
References
- US 2944062, Hoffman K, Hunger A, "Certain Alpha (1-diethylaminoethyl (2), Alpha Aryl Acetamides", issued 5 July 1960, assigned to Ciba Pharma Products Inc.
- Gross F, Turrian H (October 1957). "[Benzimidazole derivatives with strong analgesic effects]". Experientia. 13 (10): 401–3. doi:10.1007/BF02161117. PMID 13473818. S2CID 6824038.
- Vandeputte M, Van Uytfanghe K, Layle N, Germaine DS, Iula D, Stove C (12 November 2020). "Synthesis, chemical characterization, and µ-opioid receptor activity assessment of the emerging group of nitazene new synthetic opioids". Authorea. doi:10.22541/au.160520665.59016513/v1.
- Renton P, Green B, Maddaford S, Rakhit S, Andrews JS (March 2012). "NOpiates: Novel Dual Action Neuronal Nitric Oxide Synthase Inhibitors with μ-Opioid Agonist Activity". ACS Medicinal Chemistry Letters. 3 (3): 227–31. doi:10.1021/ml200268w. PMC 4025805. PMID 24900459.
- Hunger A, Kebrle J, Rossi A, Hoffmann K (October 1957). "[Synthesis of analgesically active benzimidazole derivatives with basic substitutions]" [Synthesis of analgesically active benzimidazole derivatives with basic substitutions]. Experientia. 13 (10): 400–1. doi:10.1007/BF02161116. PMID 13473817. S2CID 32179439.
- Rossi A, Hunger A, Kebrle J, Hoffmann K (1960). "Benzimidazol-Derivate und verwandte Heterocyclen. IV. Die Kondensation von o-Phenylendiamin mit α-Aryl- und γ-Aryl-acetessigester" [Benzimidazole derivatives and related heterocycles IV. The condensation of o-phenylenediamine with α-aryl and γ-aryl-acetoacetate]. Helvetica Chimica Acta (in German). 43 (4): 1046–1056. doi:10.1002/hlca.19600430413.
- Rossi A, Hunger A, Kebrle J, Hoffmann K (1960). "Benzimidazol-Derivate und verwandte Heterocyclen V. Die Kondensation von o-Phenylendiamin mit aliphatischen und alicyclischen β-Ketoestern" [Benzimidazole derivatives and related heterocycles V. The condensation of o-phenylenediamine with aliphatic and alicyclic β-keto esters]. Helvetica Chimica Acta (in German). 43 (5): 1298–1313. doi:10.1002/hlca.19600430515.
- Hunger A, Kebrle J, Rossi A, Hoffmann K (1960). "Benzimidazol-Derivate und verwandte Heterocyclen VI. Synthese von Phenyl-[1-aminoalkyl-benzimidazolyl-(2)]-essigsäure-estern und -amiden" [Benzimidazole derivatives and related Heterocycles VI. Synthesis of phenyl-[1-aminoalkyl-benzimidazolyl-(2)]-acetic acid esters and amides]. Helvetica Chimica Acta (in German). 43 (6): 1727–1733. doi:10.1002/hlca.19600430634.
- Hunger A, Kebrle J, Rossi A, Hoffmann K (1961). "Benzimidazol-Derivate und verwandte Heterocyclen VII. Synthese neuer 2-Amino-benzimidazole" [Benzimidazole Derivatives and related Heterocycles VII. Synthesis of new 2-amino-benzimidazole]. Helvetica Chimica Acta (in German). 44 (5): 1273–1282. doi:10.1002/hlca.19610440513.
- Gross F, Turrian H (October 1957). "[Benzimidazole derivatives with strong analgesic effects]" [Benzimidazole derivatives with strong analgesic effects]. Experientia. 13 (10): 401–3. doi:10.1007/BF02161117. PMID 13473818. S2CID 6824038.
- Seki T, Sasajima M, Watanbe Y, Nakajima K (March 1967). "[Studies on 2-benzimidazolethiol derivatives. N. Analgesic effect and pharmacological property of 1-(2-diethylaminoethyl)-2-(p-ethoxyphenylthio)benzimidazole hydrochloride]". Yakugaku Zasshi (in Japanese). 87 (3): 296–301. doi:10.1248/yakushi1947.87.3_296. PMID 6069375.
- Seki T (March 1967). "[Studies on 2-benzimidazolethiol derivatives. V. Structure-activity relationship on analgesic action of 1-(dialkylamino-alkyl)-2-(p-ethoxyphenylthio)benzimidazole]". Yakugaku Zasshi (in Japanese). 87 (3): 301–9. doi:10.1248/yakushi1947.87.3_301. PMID 6069376.
- Seki T, Watanabe Y (May 1969). "[Studies on 2-benzimidazolethiol derivatives. VI. Synthesis and analgesic effect of 1-(2-diethylaminoethyl)-2-(p-ethoxyphenylthio)-5-substituted benzimidazole]". Yakugaku Zasshi (in Japanese). 89 (5): 617–26. doi:10.1248/yakushi1947.89.5_617. PMID 5817995.
- Wu Z, Hruby VJ (October 2019). "Toward a Universal μ-Agonist Template for Template-Based Alignment Modeling of Opioid Ligands". ACS Omega. 4 (17): 17457–17476. doi:10.1021/acsomega.9b02244. PMC 6812133. PMID 31656918.
- Beckett AH, Casy AF (February 1965). "Analgesics and their antagonists: biochemical aspects and structure-activity relationships". Progress in Medicinal Chemistry. 4: 171–218. doi:10.1016/s0079-6468(08)70169-3. PMID 5319798.