Isophorone diamine
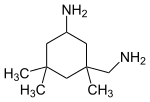
Isophorone diamine (usually shortened to IPDA) is a diamine with the formula (CH3)3C6H7(NH2)(CH2NH2). It is a colorless liquid. It is a precursor to polymers and coatings.[1]
 | |
| Names | |
|---|---|
| IUPAC name
3-(Aminomethyl)-3,5,5-trimethylcyclohexan-1-amine | |
| Other names
Isophorondiamin; IPDA | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.018.788 |
| EC Number |
|
PubChem CID |
|
| UNII | |
| UN number | 2289 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H22N2 | |
| Molar mass | 170.300 g·mol−1 |
| Appearance | Colourless liquid |
| Density | 0.922 |
| Melting point | 10 °C (50 °F; 283 K) |
| Boiling point | 247 °C (477 °F; 520 K) |
| Very good | |
Refractive index (nD) |
1.4880 |
| Hazards | |
| GHS pictograms | 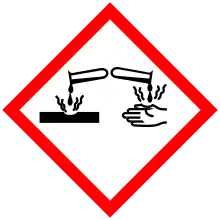  |
| GHS Signal word | Danger |
| H302, H312, H314, H317, H318, H412 | |
| P260, P261, P264, P270, P272, P273, P280, P301+312, P301+330+331, P302+352, P303+361+353, P304+340, P305+351+338, P310, P312, P321, P322, P330, P333+313, P363, P405, P501 | |
| Flash point | 117°C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Production
It is usually produced as a mixture of the cis- and trans-isomers. It is produced by hydrocyanation of isophorone followed by reductive amination and hydrogenation of the nitrile.[1]
Uses
IPDA is used as a precursor in the manufacture of isophorone diisocyanate by phosgenation.[2]
Like other diamines, it is a curing agent for epoxy resins. When used in coatings applications the higher cost compared to other amines is justified by the enhanced UV stability and thus lower yellowing tendency. In the production of Advanced composite materials (engineering) its higher cost compared to other amines is less critical as performance is the key criteria.[3][4] Cycloaliphatic amines such as IPDA also are known to have lower yellowing tendency than other amines and are thus used in coatings applications where this feature is important for aesthetics.
References
- Hardo Siegel, Manfred Eggersdorfer (2005). "Ketones". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a15_077.CS1 maint: uses authors parameter (link)
- The polyurethanes book. Randall, David, Lee, Steve, 1941-. [Everberg, Belgium]: [Huntsman Polyurethanes]. 2002. ISBN 0470850418. OCLC 50479333.CS1 maint: others (link)
- Pilato, Louis A.; Michno, Michael J. (1994-11-04). Advanced Composite Materials. Springer Science & Business Media. ISBN 9783540575634.
- "Cycloaliphatic Amines - Hexion.com". www.hexion.com. Retrieved 2018-08-17.