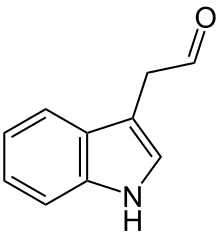
Indole-3-acetaldehyde
Indole-3-acetaldehyde belongs to the class of organic compounds known as indoles. These are compounds containing an indole moiety, which consists of pyrrole ring fused to benzene to form 2,3-benzopyrrole.
 | |
| Names | |
|---|---|
| IUPAC name
2-(1H-indol-3-yl)acetaldehyde | |
| Other names
Indoleacetaldehyde; 1H-Indole-3-acetaldehyde; 2-(indol-3-yl)acetaldehyde; Indole-3-acetaldehyde; Indoleacetaldehyde; 1H-indol-3-ylacetaldehyde; 2-(3-Indolyl)acetaldehyde; indol-3-ylacetaldehyde; Tryptaldehyde | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
| MeSH | C001655 |
PubChem CID |
|
| |
| |
| Properties | |
| C10H9NO | |
| Molar mass | 159.188 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Indole-3-acetaldehyde is a substrate for retina-specific copper amine oxidase, aldehyde dehydrogenase X (mitochondrial), amine oxidase B, amiloride-sensitive amine oxidase, aldehyde dehydrogenase (mitochondrial), fatty aldehyde dehydrogenase, 4-trimethylaminobutyraldehyde dehydrogenase, aldehyde dehydrogenase (dimeric NADP-preferring), aldehyde dehydrogenase family 7 member A1, amine oxidase A, aldehyde dehydrogenase 1A3 and membrane copper amine oxidase.[1]
References
- Nutaratat P, Srisuk N, Arunrattiyakorn P, Limtong S (2016). "Indole-3-acetic acid biosynthetic pathways in the basidiomycetous yeast Rhodosporidium paludigenum". Arch Microbiol. 198 (5): 429–37. doi:10.1007/s00203-016-1202-z.
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.