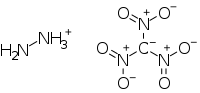
Hydrazinium nitroformate
Hydrazinium nitroformate (HNF) is a salt of hydrazine and nitroform (trinitromethane).[1][2] It has the molecular formula [H2NNH3]+[C(NO2)3]−[3] and is soluble in most solvents.
 | |
| Names | |
|---|---|
| Other names
Hydrazine nitroform; HNF | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
| |
| |
| Properties | |
| CH5N5O6 | |
| Molar mass | 183.080 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Hydrazinium nitroformate is an energetic oxidizer. Research is being conducted at the European Space Agency to investigate its use in solid rocket propellants.[4][5] It tends to produce propellants which burn very rapidly and with very high combustion efficiency. Its high energy leads to high specific impulse propellants. It is currently an expensive research chemical available only in limited quantities. A disadvantage of HNF is its limited thermal stability.
References
- H. F. R. Schoyer, W. H. M. Welland-Veltman, J. Louwers, P. A. O. G. Korting, A. E. D. M. van der Heijden, H. L. J. Keizers, and R. P. van den Berg (2002). "Overview of the Development of Hydrazinium Nitroformate". Journal of Propulsion and Power. 18 (1): 131–137. doi:10.2514/2.5908.CS1 maint: multiple names: authors list (link)
- P. S. Dendage, D. B. Sarwade, S. N. Asthana & H. Singh (2001). "Hydrazinium nitroformate (HNF) and HNF based propellants: A review". Journal of Energetic Materials. 19 (1): 41–78. doi:10.1080/07370650108219392.CS1 maint: multiple names: authors list (link)
- Dickens, Brian (1967). "Crystal structure of hydrazine nitroform [N2H5+C(NO2)3−]". Chemical Communications (5): 246–247. doi:10.1039/c19670000246.
- "Preparing for the Future". Vol. 6 no. 1. European Space Agency. March 1996. Cite magazine requires
|magazine=(help) - Tydon, Walter (31 July 1970). Minimum Cost Design Launch Vehicle Design/Costing Study TOR-0059(6526-01)2. vol. 2, Background Studies. The Aerospace Corporation. pp. 4–13, 4–14.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.