Hexafluorocyclobutene
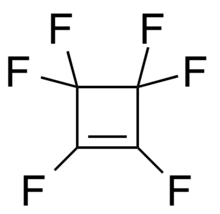
Hexafluorocyclobutene is the organofluorine compound with the formula (CF2)2(CF)2. A colorless gas, it is a precursor to a variety of compounds, including squaric acid.[1] Hexafluorocyclobutene is prepared in two steps from chlorotrifluoroethylene. The thermal dimerization gives 1,2-dichloro-1,2,3,3,4,4-hexafluorocyclobutane.[2] Dichlorination of the latter gives hexafluorocyclobutene:[3]
- C4F6Cl2 + Zn C4F6 + ZnCl2
 | |
| Names | |
|---|---|
| Other names
1,2,3,3,4,4-hexafluorocyclobutene, perfluorocyclobutene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4F6 | |
| Molar mass | 162.034 g·mol−1 |
| Appearance | colorless gas |
| Melting point | −60 °C (−76 °F; 213 K) |
| Boiling point | 5.5 °C (41.9 °F; 278.6 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
See also
Safety
Reminiscent of perfluoroisobutene, hexafluorocyclobutene is quite toxic with an LD = 6000 mg/min/m−3 (mice).[4]
References
- David M. Lemal, Xudong Chen (2005). "Fluorinated Cyclobutanes and Their Derivatives". In Zvi Rappoport; Joel F. Liebman (eds.). The Chemistry of Cyclobutanes. PATAI'S Chemistry of Functional Groups. pp. 955–1029. doi:10.1002/0470864028.ch21. ISBN 0470864001.
- Buxton, M. W.; Ingram, D. W.; Smith, F.; Stacey, M.; Tatlow, J. C. (1952). "The High-Temperature Dimerisation of Chlorotrifluoroethylene". Journal of the Chemical Society (Resumed): 3830. doi:10.1039/JR9520003830.
- Fuller, G.; Tatlow, J. C. (1961). "Some Isomeric Hexafluorocyclobutanes and Pentafluorocyclobutenes". Journal of the Chemical Society (Resumed): 3198. doi:10.1039/JR9610003198.
- Timperley, Christopher M. (2000). "Highly-toxic fluorine compounds". Fluorine Chemistry at the Millennium. pp. 499–538. doi:10.1016/B978-008043405-6/50040-2. ISBN 9780080434056.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.