Hexaammineplatinum(IV) chloride
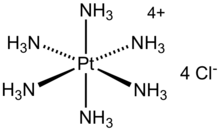
Hexaammineplatinum(IV) chloride is the chemical compound with the formula [Pt(NH3)6]Cl4. It is the chloride salt of the metal ammine complex [Pt(NH3)6]4+. The cation features six ammonia (called ammines in coordination chemistry) ligands attached to the platinum(IV) ion. It is a white, water soluble solid.
 | |
| Names | |
|---|---|
| IUPAC name
Hexaammineplatinum(IV) chloride | |
| Other names
Platinum hexammine tetrachloride | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
| |
| |
| Properties | |
| Cl4H18N6Pt | |
| Molar mass | 439.07 g·mol−1 |
| Appearance | white solid |
| Melting point | decomposes |
| Solubility | soluble in NH3 |
| Structure | |
| octahedral | |
| 0 D | |
| Related compounds | |
Other cations |
[Ni(NH3)6]Cl2 [Co(NH3)6]Cl3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Properties and structure
Typical for platinum(IV) complexes, [Pt(NH3)6]4+ is diamagnetic and kinetically inert, e.g. unaffected by strong acids. The cation obeys the 18-electron rule. It is prepared by treatment of methylamine complex [Pt(NH2CH3)4Cl2]Cl2 with ammonia.[1]
The complex [Pt(NH3)6]4+ is a rare example of a tetracationic ammine complex. Its conjugate bases [Pt(NH3)5NH2]3+ and [Pt(NH3)4(NH2)2]2+ have been characterized.[2]
References
- L. N. Essen (1974). "Hexaammineplatinum(IV) Chloride". Inorganic Syntheses. Inorganic Syntheses. 15. p. 93. doi:10.1002/9780470132463.ch21. ISBN 9780470132463.
- B. Klein, L. Heck (1975). "Deprotonierung von Komplexliganden. I. Amidoamminkomplexe des Platin(IV)". Zeitschrift für anorganische Chemie. 416 (3): 269–284. doi:10.1002/zaac.19754160311.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.