Gut–brain axis
The gut–brain axis is the biochemical signaling that takes place between the gastrointestinal tract (GI tract) and the central nervous system (CNS).[1] The term "gut–brain axis" is occasionally used to refer to the role of the gut flora in the interplay as well, whereas the term "microbiota–gut–brain (MGB or BGM) axis" explicitly includes the role of gut flora in the biochemical signaling events that take place between the GI tract and CNS.[1][2][3]
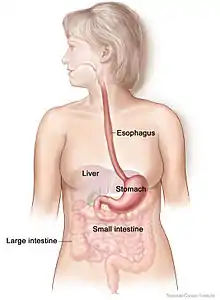
Broadly defined, the gut–brain axis includes the central nervous system, neuroendocrine and neuroimmune systems, including the hypothalamic–pituitary–adrenal axis (HPA axis), sympathetic and parasympathetic arms of the autonomic nervous system, including the enteric nervous system and the vagus nerve, and the gut microbiota.[1][3] The first of the brain–gut interactions shown, was the cephalic phase of digestion, in the release of gastric and pancreatic secretions in response to sensory signals, such as the smell and sight of food. This was first demonstrated by Pavlov.[4][5]
Interest in the field was sparked by a 2004 study showing that germ-free (GF) mice showed an exaggerated HPA axis response to stress compared to non-GF laboratory mice.[1]
As of October 2016, most of the work done on the role of gut flora in the gut–brain axis had been conducted in animals, or on characterizing the various neuroactive compounds that gut flora can produce. Studies with humans – measuring variations in gut flora between people with various psychiatric and neurological conditions or when stressed, or measuring effects of various probiotics (dubbed "psychobiotics" in this context) – had generally been small and were just beginning to be generalized.[6] Whether changes to gut flora are a result of disease, a cause of disease, or both in any number of possible feedback loops in the gut–brain axis, remained unclear.[7][1]
Gut flora

The gut flora is the complex community of microorganisms that live in the digestive tracts of humans and other animals. The gut metagenome is the aggregate of all the genomes of gut microbiota.[8] The gut is one niche that human microbiota inhabit.[9]
In humans, the gut microbiota has the largest quantity of bacteria and the greatest number of species, compared to other areas of the body.[10] In humans, the gut flora is established at one to two years after birth; by that time, the intestinal epithelium and the intestinal mucosal barrier that it secretes have co-developed in a way that is tolerant to, and even supportive of, the gut flora and that also provides a barrier to pathogenic organisms.[11][12]
The relationship between gut flora and humans is not merely commensal (a non-harmful coexistence), but rather a mutualistic relationship.[9] Human gut microorganisms benefit the host by collecting the energy from the fermentation of undigested carbohydrates and the subsequent absorption of short-chain fatty acids (SCFAs), acetate, butyrate, and propionate.[10][13] Intestinal bacteria also play a role in synthesizing vitamin B and vitamin K as well as metabolizing bile acids, sterols, and xenobiotics.[9][13] The systemic importance of the SCFAs and other compounds they produce are like hormones and the gut flora itself appears to function like an endocrine organ;[13] dysregulation of the gut flora has been correlated with a host of inflammatory and autoimmune conditions.[10][14]
The composition of human gut flora changes over time, when the diet changes, and as overall health changes.[10][14]
Tryptophan metabolism by human gastrointestinal microbiota ()
|
Enteric nervous system
The enteric nervous system is one of the main divisions of the nervous system and consists of a mesh-like system of neurons that governs the function of the gastrointestinal system; it has been described as a "second brain" for several reasons. The enteric nervous system can operate autonomously. It normally communicates with the central nervous system (CNS) through the parasympathetic (e.g., via the vagus nerve) and sympathetic (e.g., via the prevertebral ganglia) nervous systems. However, vertebrate studies show that when the vagus nerve is severed, the enteric nervous system continues to function.[19]
In vertebrates, the enteric nervous system includes efferent neurons, afferent neurons, and interneurons, all of which make the enteric nervous system capable of carrying reflexes in the absence of CNS input. The sensory neurons report on mechanical and chemical conditions. Through intestinal muscles, the motor neurons control peristalsis and churning of intestinal contents. Other neurons control the secretion of enzymes. The enteric nervous system also makes use of more than 30 neurotransmitters, most of which are identical to the ones found in CNS, such as acetylcholine, dopamine, and serotonin. More than 90% of the body's serotonin lies in the gut, as well as about 50% of the body's dopamine; the dual function of these neurotransmitters is an active part of gut–brain research.[20][21][22]
The first of the gut–brain interactions was shown to be between the sight and smell of food and the release of gastric secretions, known as the cephalic phase, or cephalic response of digestion.[4][5]
Gut–brain integration
The gut–brain axis, a bidirectional neurohumoral communication system, is important for maintaining homeostasis and is regulated through the central and enteric nervous systems and the neural, endocrine, immune, and metabolic pathways, and especially including the hypothalamic–pituitary–adrenal axis (HPA axis).[1] That term has been expanded to include the role of the gut flora as part of the "microbiome-gut-brain axis", a linkage of functions including the gut flora.[1][3][2]
Interest in the field was sparked by a 2004 study (Nobuyuki Sudo and Yoichi Chida) showing that germ-free mice (genetically homogeneous laboratory mice, birthed and raised in an antiseptic environment) showed an exaggerated HPA axis response to stress, compared to non-GF laboratory mice.[1]
The gut flora can produce a range of neuroactive molecules, such as acetylcholine, catecholamines, γ-aminobutyric acid, histamine, melatonin, and serotonin, which are essential for regulating peristalsis and sensation in the gut.[23] Changes in the composition of the gut flora due to diet, drugs, or disease correlate with changes in levels of circulating cytokines, some of which can affect brain function.[23] The gut flora also release molecules that can directly activate the vagus nerve, which transmits information about the state of the intestines to the brain.[23]
Likewise, chronic or acutely stressful situations activate the hypothalamic–pituitary–adrenal axis, causing changes in the gut flora and intestinal epithelium, and possibly having systemic effects.[23] Additionally, the cholinergic anti-inflammatory pathway, signaling through the vagus nerve, affects the gut epithelium and flora.[23] Hunger and satiety are integrated in the brain, and the presence or absence of food in the gut and types of food present also affect the composition and activity of gut flora.[23]
That said, most of the work that has been done on the role of gut flora in the gut–brain axis has been conducted in animals, including the highly artificial germ-free mice. As of 2016, studies with humans measuring changes to gut flora in response to stress, or measuring effects of various probiotics, have generally been small and cannot be generalized; whether changes to gut flora are a result of disease, a cause of disease, or both in any number of possible feedback loops in the gut–brain axis, remains unclear.[7]
The history of ideas about a relationship between the gut and the mind dates from the nineteenth century. The concepts of dyspepsia and neurasthenia gastrica referred to the influence of the gut on human emotions and thoughts.[24][25]
Gut-brain-skin axis
A unifying theory that tied gastrointestinal mechanisms to anxiety, depression, and skin conditions such as acne was proposed as early as 1930.[26] In a paper in 1930, it was proposed that emotional states might alter normal intestinal flora which could lead to increased intestinal permeability and therefore contribute to systemic inflammation. Many aspects of this theory have been validated since then. Gut microbiota and oral probiotics have been found to influence systemic inflammation, oxidative stress, glycemic control, tissue lipid content, and mood.[27]
Research
Probiotics
A 2016 systematic review of laboratory animal studies and preliminary human clinical trials using commercially available strains of probiotic bacteria found that certain species of the Bifidobacterium and Lactobacillus genera (i.e., B. longum, B. breve, B. infantis, L. helveticus, L. rhamnosus, L. plantarum, and L. casei) had the most potential to be useful for certain central nervous system disorders.[28]
Anxiety and mood disorders
As of 2018 work on the relationship between gut flora and anxiety disorders and mood disorders, as well as attempts to influence that relationship using probiotics or prebiotics (called "psychobiotics"), was at an early stage, with insufficient evidence to draw conclusions about a causal role for gut flora changes in these conditions, or about the efficacy of any probiotic or prebiotic treatment.[29][7]
People with anxiety and mood disorders tend to have gastrointestinal problems; small studies have been conducted to compare the gut flora of people with major depressive disorder and healthy people, but those studies have had contradictory results.[7]
Much interest was generated in the potential role of gut flora in anxiety disorders, and more generally in the role of gut flora in the gut–brain axis, by studies published in 2004 showing that germ-free mice have an exaggerated HPA axis response to stress caused by being restrained, which was reversed by colonizing their gut with a Bifidobacterium species.[2] Studies looking at maternal separation for rats shows neonatal stress leads to long-term changes in the gut microbiota such as its diversity and composition, which also led to stress and anxiety-like behavior.[30] Additionally, while much work had been done as of 2016 to characterize various neurotransmitters known to be involved in anxiety and mood disorders that gut flora can produce (for example, Escherichia, Bacillus, and Saccharomyces species can produce noradrenalin; Candida, Streptococcus, and Escherichia species can produce serotonin, etc.) the interrelationships and pathways by which the gut flora might affect anxiety in humans were unclear.[8]
In one study, germ-free mice underwent fecal transplants with microbes from humans with or without major depressive disorder (MDD). Mice with microbes from humans with MDD displayed more behaviors associated with anxiety and depression than mice transplanted with microbes from humans without MDD. The taxonomic composition of microbiota between depressed patients and healthy patients, as well as between the respective mice, also differed.[31] Germ-free mice in another study also displayed behaviors associated with anxiety and depression as compared to mice with normal microbiota, and had higher levels of corticosterone after exposure to behavioral tests.[32] Using rodents in microbiome and mental health studies allows researchers to compare behavior and microbial composition of rodents to humans, ideally to elucidate therapeutic application for mental disorders.
Additionally, there is a link between the gut microbiome, mood disorders and anxiety, and sleep. The microbial composition of the gut microbiome changes depending on the time of day, meaning that throughout the day, the gut is exposed to varying metabolites produced by the microbes active during that time. These time-dependent microbial changes are associated with differences in the transcription of circadian clock genes involved in circadian rhythm. One mouse study showed that altering clock gene transcription by disrupting circadian rhythm, such as through sleep deprivation, potentially has a direct effect on the composition of the gut microbiome.[33] Another study found that mice that could not produce the CLOCK protein, made by a clock gene, were more likely to develop depression.[33] Stress and sleep disturbances can lead to greater gut mucosal permeability via activation of the HPA axis. This in turn causes immune inflammatory responses that contribute to the development of illnesses that cause depression and anxiety.[33]
Autism
Around 70% of people with autism also have gastrointestinal problems, and autism is often diagnosed at the time that the gut flora becomes established, indicating that there may be a connection between autism and gut flora.[34] Some studies have found differences in the gut flora of children with autism compared with children without autism – most notably elevations in the amount of Clostridium in the stools of children with autism compared with the stools of the children without[35] – but these results have not been consistently replicated.[34] Many of the environmental factors thought to be relevant to the development of autism would also affect the gut flora, leaving open the question of whether specific developments in the gut flora drive the development of autism or whether those developments happen concurrently.[3][34] As of 2016, studies with probiotics had only been conducted with animals; studies of other dietary changes to treat autism have been inconclusive.[7]
Parkinson's disease
As of 2015, one study had been conducted comparing the gut flora of people with Parkinson's disease to healthy controls; in that study people with Parkinson's had lower levels of Prevotellaceae and people with Parkinson's who had higher levels of Enterobacteriaceae had more clinically severe symptoms; the authors of the study drew no conclusions about whether gut flora changes were driving the disease or vice versa.[3]
References
- Sudo, N; Chida, Y; Aiba, Y (2004). "Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice". J Physiol. 558 (1): 263–275. doi:10.1113/jphysiol.2004.063388. PMC 1664925. PMID 15133062. cited in: Wang, Y; Kasper, LH (May 2014). "The role of microbiome in central nervous system disorders". Brain Behav Immun. 38: 1–12. doi:10.1016/j.bbi.2013.12.015. PMC 4062078. PMID 24370461.
- Mayer, EA; Knight, R; Mazmanian, SK; et al. (2014). "Gut microbes and the brain: paradigm shift in neuroscience". J Neurosci. 34 (46): 15490–15496. doi:10.1523/JNEUROSCI.3299-14.2014. PMC 4228144. PMID 25392516.
- Dinan, T.G; Cryan, 2015 (2015). "The impact of gut microbiota on brain and behavior: implications for psychiatry". Curr Opin Clin Nutr Metab Care. 18 (6): 552–558. doi:10.1097/MCO.0000000000000221. PMID 26372511. S2CID 21424690.CS1 maint: numeric names: authors list (link)
- Filaretova, L; Bagaeva, T (2016). "The Realization of the Brain–Gut Interactions with Corticotropin-Releasing Factor and Glucocorticoids". Current Neuropharmacology. 14 (8): 876–881. doi:10.2174/1570159x14666160614094234. PMC 5333583. PMID 27306034.
- Smeets, PA; Erkner, A; de Graaf, C (November 2010). "Cephalic phase responses and appetite". Nutrition Reviews. 68 (11): 643–55. doi:10.1111/j.1753-4887.2010.00334.x. PMID 20961295.
- Wang, Huiying; Lee, In-Seon; Braun, Christoph; Enck, Paul (October 2016). "Effect of Probiotics on Central Nervous System Functions in Animals and Humans: A Systematic Review". J Neurogastroenterol Motil. 22 (4): 589–605. doi:10.5056/jnm16018. PMC 5056568. PMID 27413138.
- Schneiderhan, J; Master-Hunter, T; Locke, A (2016). "Targeting gut flora to treat and prevent disease". J Fam Pract. 65 (1): 34–8. PMID 26845162. Archived from the original on 2016-08-15. Retrieved 2016-06-25.
- Saxena, R.; Sharma, V.K (2016). "A Metagenomic Insight Into the Human Microbiome: Its Implications in Health and Disease". In D. Kumar; S. Antonarakis (eds.). Medical and Health Genomics. Elsevier Science. p. 117. doi:10.1016/B978-0-12-420196-5.00009-5. ISBN 978-0-12-799922-7.
- Sherwood, Linda; Willey, Joanne; Woolverton, Christopher (2013). Prescott's Microbiology (9th ed.). New York: McGraw Hill. pp. 713–721. ISBN 978-0-07-340240-6. OCLC 886600661.
- Quigley, EM (2013). "Gut bacteria in health and disease". Gastroenterol Hepatol (N Y). 9 (9): 560–9. PMC 3983973. PMID 24729765.
- Sommer, F; Bäckhed, F (Apr 2013). "The gut microbiota--masters of host development and physiology". Nat Rev Microbiol. 11 (4): 227–38. doi:10.1038/nrmicro2974. PMID 23435359. S2CID 22798964.
- Faderl, M; et al. (Apr 2015). "Keeping bugs in check: The mucus layer as a critical component in maintaining intestinal homeostasis". IUBMB Life. 67 (4): 275–85. doi:10.1002/iub.1374. PMID 25914114. S2CID 25878594.
- Clarke, G; et al. (Aug 2014). "Minireview: Gut microbiota: the neglected endocrine organ". Mol Endocrinol. 28 (8): 1221–38. doi:10.1210/me.2014-1108. PMC 5414803. PMID 24892638.
- Shen, S; Wong, CH (Apr 2016). "Bugging inflammation: role of the gut microbiota". Clin Transl Immunol. 5 (4): e72. doi:10.1038/cti.2016.12. PMC 4855262. PMID 27195115.
- Zhang LS, Davies SS (April 2016). "Microbial metabolism of dietary components to bioactive metabolites: opportunities for new therapeutic interventions". Genome Med. 8 (1): 46. doi:10.1186/s13073-016-0296-x. PMC 4840492. PMID 27102537.
Lactobacillus spp. convert tryptophan to indole-3-aldehyde (I3A) through unidentified enzymes [125]. Clostridium sporogenes convert tryptophan to IPA [6], likely via a tryptophan deaminase. ... IPA also potently scavenges hydroxyl radicals
Table 2: Microbial metabolites: their synthesis, mechanisms of action, and effects on health and disease
Figure 1: Molecular mechanisms of action of indole and its metabolites on host physiology and disease - Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G (March 2009). "Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites". Proc. Natl. Acad. Sci. U.S.A. 106 (10): 3698–3703. doi:10.1073/pnas.0812874106. PMC 2656143. PMID 19234110.
Production of IPA was shown to be completely dependent on the presence of gut microflora and could be established by colonization with the bacterium Clostridium sporogenes.
IPA metabolism diagram - "3-Indolepropionic acid". Human Metabolome Database. University of Alberta. Retrieved 12 June 2018.
Indole-3-propionate (IPA), a deamination product of tryptophan formed by symbiotic bacteria in the gastrointestinal tract of mammals and birds. 3-Indolepropionic acid has been shown to prevent oxidative stress and death of primary neurons and neuroblastoma cells exposed to the amyloid beta-protein in the form of amyloid fibrils, one of the most prominent neuropathologic features of Alzheimer's disease. 3-Indolepropionic acid also shows a strong level of neuroprotection in two other paradigms of oxidative stress. (PMID 10419516) ... More recently it has been found that higher indole-3-propionic acid levels in serum/plasma are associated with reduced likelihood of type 2 diabetes and with higher levels of consumption of fiber-rich foods (PMID 28397877)
Origin: • Endogenous • Microbial - Chyan YJ, Poeggeler B, Omar RA, Chain DG, Frangione B, Ghiso J, Pappolla MA (July 1999). "Potent neuroprotective properties against the Alzheimer beta-amyloid by an endogenous melatonin-related indole structure, indole-3-propionic acid". J. Biol. Chem. 274 (31): 21937–21942. doi:10.1074/jbc.274.31.21937. PMID 10419516.
[Indole-3-propionic acid (IPA)] has previously been identified in the plasma and cerebrospinal fluid of humans, but its functions are not known. ... In kinetic competition experiments using free radical-trapping agents, the capacity of IPA to scavenge hydroxyl radicals exceeded that of melatonin, an indoleamine considered to be the most potent naturally occurring scavenger of free radicals. In contrast with other antioxidants, IPA was not converted to reactive intermediates with pro-oxidant activity.
- Li, Ying; Owyang, Chung (September 2003). "Musings on the Wanderer: What's New in Our Understanding of Vago-Vagal Reflexes? V. Remodeling of vagus and enteric neural circuitry after vagal injury". American Journal of Physiology. Gastrointestinal and Liver Physiology. 285 (3): G461–9. doi:10.1152/ajpgi.00119.2003. PMID 12909562.
- Pasricha, Pankaj Jay. "Stanford Hospital: Brain in the Gut – Your Health".
- Martinucci, I; et al. (2015). "Genetics and pharmacogenetics of aminergic transmitter pathways in functional gastrointestinal disorders". Pharmacogenomics. 16 (5): 523–39. doi:10.2217/pgs.15.12. PMID 25916523.
- Smitka, K; et al. (2013). "The role of "mixed" orexigenic and anorexigenic signals and autoantibodies reacting with appetite-regulating neuropeptides and peptides of the adipose tissue-gutbrain axis: relevance to food intake and nutritional status in patients with anorexia nervosa and bulimia nervosa". Int J Endocrinol. 2013: 483145. doi:10.1155/2013/483145. PMC 3782835. PMID 24106499.
- Petra, AI; et al. (May 2015). "Gut-Microbiota-Brain Axis and Its Effect on Neuropsychiatric Disorders With Suspected Immune Dysregulation". Clin. Ther. 37 (5): 984–95. doi:10.1016/j.clinthera.2015.04.002. PMC 4458706. PMID 26046241.
- Manon Mathias and Alison M. Moore (eds), Gut Feeling and Digestive Health in Nineteenth-Century Literature, History and Culture. New York: Palgrave, 2018. ISBN 9780230303454
- Alison M. Moore, Manon Mathias and Jørgen Valeur, Microbial Ecology in Health and Disease, Volume 30 (1), Special issue on the Gut–Brain Axis in History and Culture, 2019
- Stokes; Pillsbury (December 1930). "The effect on the skin of emotional and nervous states: Theoretical and practical consideration of a gastro-intestinal mechanism". Cite journal requires
|journal=(help) - Bowe, W. P.; Logan, A. C. (2011). "Acne vulgaris, probiotics and the gut-brain-skin axis - back to the future?". Gut Pathogens. 3 (1): 1. doi:10.1186/1757-4749-3-1. PMC 3038963. PMID 21281494.
- Wang H, Lee IS, Braun C, Enck P (July 2016). "Effect of probiotics on central nervous system functions in animals and humans - a systematic review". J. Neurogastroenterol. Motil. 22 (4): 589–605. doi:10.5056/jnm16018. PMC 5056568. PMID 27413138.
We reviewed the effect of probiotics on the central nervous system in randomized controlled trials in animals and humans, and analyzed the possibility of translating animal models to human studies because few human studies have been conducted to date. According to the qualitative analyses of current studies, we can provisionally draw the conclusion that B. longum, B. breve, B. infantis, L. helveticus, L. rhamnosus, L. plantarum, and L. casei were most effective in improving CNS function, including psychiatric disease-associated functions (anxiety, depression, mood, stress response) and memory abilities.
- Sarkar, Amar; Lehto, Soili M.; Harty, Siobhán; Dinan, Timothy G.; Cryan, John F.; Burnet, Philip W.J. (2016). "Psychobiotics and the Manipulation of Bacteria–Gut–Brain Signals". Trends in Neurosciences. 39 (11): 763–781. doi:10.1016/j.tins.2016.09.002. ISSN 0166-2236. PMC 5102282. PMID 27793434.
- Foster, J.A.; McVey Neufelt, K.A. (2013). "Gut–brain axis: how the microbiome influences anxiety and depression". Trends in Neurosciences. 36 (5): 305–312. doi:10.1016/j.tins.2013.01.005. PMID 23384445. S2CID 14841718.
- Zheng, P; Zeng, B; Zhou, C; Liu, M; Fang, Z; Xu, X; Zeng, L; Chen, J; Fan, S (2016-04-12). "Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host's metabolism". Molecular Psychiatry. 21 (6): 786–796. doi:10.1038/mp.2016.44. ISSN 1359-4184. PMID 27067014. S2CID 9636895.
- Crumeyrolle-Arias, Michèle; Jaglin, Mathilde; Bruneau, Aurélia; Vancassel, Sylvie; Cardona, Ana; Daugé, Valérie; Naudon, Laurent; Rabot, Sylvie (April 2014). "Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats". Psychoneuroendocrinology. 42: 207–217. doi:10.1016/j.psyneuen.2014.01.014. ISSN 0306-4530. PMID 24636517. S2CID 33589074.
- Li, Yuanyuan; Hao, Yanli; Fan, Fang; Zhang, Bin (2018-12-05). "The Role of Microbiome in Insomnia, Circadian Disturbance and Depression". Frontiers in Psychiatry. 9. doi:10.3389/fpsyt.2018.00669. ISSN 1664-0640. PMC 6290721. PMID 30568608.
- Buie, T (May 2015). "Potential Etiologic Factors of Microbiome Disruption in Autism". Clin. Ther. 37 (5): 976–83. doi:10.1016/j.clinthera.2015.04.001. PMID 26046240.
- Chen, X; D'Souza, R; Hong, ST (2013). "The role of gut microbiota in the gut–brain axis: current challenges and perspectives". Protein & Cell. 4 (6): 403–14. doi:10.1007/s13238-013-3017-x. PMC 4875553. PMID 23686721.
