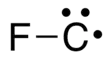Fluoromethylidyne
Fluoromethylidyne is not a stable chemical species but a metastable radical containing one highly reactive carbon atom bound to one fluorine atom with the formula CF.[1] The carbon atom has a lone-pair and a single unpaired (radical) electron in the ground state.[2]
| |||
| Names | |||
|---|---|---|---|
IUPAC name
| |||
Other names
| |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| |||
| |||
| Properties | |||
| CF• | |||
| Molar mass | 31.0091 g mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Ground-state fluoromethylidyne radicals can be produced by the ultraviolet photodissociation of dibromodifluoromethane at 248 nanometer wavelength.[3]
It readily and irreversibly dimerises to difluoroacetylene, also known as difluoroethyne, perfluoroacetylene, or di- or perfluoroethylyne. Under certain conditions it can hexamerise to hexafluorobenzene.
See also
References
- F. J. Grieman, A. T. Droege, and P. C. Engelking (March 1983). "The a4Σ−–X2Π transition in CF: A measurement of the term energy and bond length of a fluoromethylidyne metastable". Journal of Chemical Physics. 78 (5): 2248–2254. doi:10.1063/1.445070.CS1 maint: uses authors parameter (link)
- Ruzsicska, B. P.; Jodhan, A.; Choi; H. K. J., Strausz, O. P.; Bell, T. N. (1983). "Chemistry of carbynes: reaction of CF, CCl, and CBr with alkenes". J. Am. Chem. Soc. 105: 2489–2490. doi:10.1021/ja00346a072.CS1 maint: uses authors parameter (link)
- J. Peeters; J. Van Hoeymissen; S. Vanhaelemeersch; D. Vermeylen (February 1992). "Absolute rate constant measurements of CF(X2Π) reactions. 1. Reactions with O2, F2, Cl2 and NO". Journal of Physical Chemistry. 96 (3): 1257–1263. doi:10.1021/j100182a043.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.