Fischer–Hepp rearrangement
The Fischer–Hepp rearrangement is a rearrangement reaction in which an aromatic N-nitroso or nitrosamine converts to a carbon nitroso compound:[1][2]
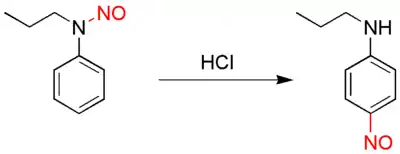
Fischer-Hepp rearrangement
| Fischer-Hepp rearrangement | |
|---|---|
| Named after | Otto Fischer Eduard Hepp |
| Reaction type | Rearrangement reaction |
| Identifiers | |
| RSC ontology ID | RXNO:0000095 |
This organic reaction was first described by the German chemist Otto Philipp Fischer (1852–1932 ) and Eduard Hepp (June 11, 1851 – June 18, 1917) [3] in 1886, and is of importance because para-NO secondary anilines cannot be prepared in a direct reaction.
The rearrangement reaction takes place by reacting the nitrosamine precursor with hydrochloric acid. The chemical yield is generally good under these conditions, but often much poorer if a different acid is used. The exact reaction mechanism is unknown but there is evidence suggesting an intramolecular reaction.
See also
References
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.