Ex-Rad
Ex-Rad (or Ex-RAD; recilisib sodium (INN, USAN); development code ON 01210.Na) is an experimental drug being developed by Onconova Therapeutics and the U.S. Department of Defense.[1] It is being studied as a radiation protection agent.[2] Chemically, it is the sodium salt of 4-carboxystyryl-4-chlorobenzylsulfone.[3]
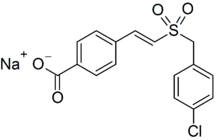 | |
| Names | |
|---|---|
| IUPAC name
Sodium 4-[(E)-2-[(4-chlorophenyl)methylsulfonyl]ethenyl]benzoate | |
| Identifiers | |
3D model (JSmol) |
|
| KEGG | |
PubChem CID |
|
| UNII | |
| |
| Properties | |
| C16H12ClNaO4S | |
| Molar mass | 358.77 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Clinical trials
The results of two Phase I clinical studies in healthy human volunteers indicate that subcutaneously injected Ex-Rad is safe and well tolerated, with "no evidence of systemic side effects".[4] A study in mice demonstrated the efficacy of Ex-Rad by increasing the survival rate of mice exposed to typically lethal whole-body irradiation. The study tested oral and parenteral administration of Ex-Rad for both pre- and post-exposure radiomitigation.[1]
Research on Ex-Rad has involved collaboration with the Armed Forces Radiobiology Research Institute (AFRRI), the Department of Biochemistry and Molecular & Cellular Biology at Georgetown University, Long Island University's Arnold & Marie Schwartz College of Pharmacy, and the Department of Oncological Sciences at the Mt. Sinai School of Medicine.[1]
Mechanism of action
Onconova reports that Ex-Rad protects cells exposed to radiation against DNA damage, and that the drug's mechanism of action does not involve scavenging free radicals or arresting the cell cycle. Instead, they claim it employs a "novel mechanism" involving "intracellular signaling, damage sensing, and DNA repair pathways".[4] Ex-RAD is a chlorobenzylsulfone derivative that works after free radicals have damaged DNA. Onconova CEO Ramesh Kumar believes this is a better approach than trying to scavenge free radicals. “Free radicals are very short-lived, and so the window of opportunity to give a drug is very narrow,” he says. In cell and animal models, Ex-RAD protects hematopoietic and gastrointestinal tissues from radiation injury when given either before or after exposure.[5]
See also
- Entolimod
- CBLB502 a compound being studied for its ability to suppresses apoptotic cell death in hematopoietic and gastrointestinal cells.[5]
- Amifostine (WR2721), the first selective-target and broad-spectrum radioprotector, upregulates DNA repair[6]
- Filgrastim (Neupogen), a hematopoietic countermeasure of acute radiation syndrome (ARS)
- Pegfilgrastim (Neulasta), longer acting than its parent, filgrastim
- Sargramostim (leukine), similar in use to filgrastim
- N-Acetylcysteine, protects against DNA damage, suggested to be comparable to amifostine[7][8][9][10]
- Thrombomodulin[11]
- Activated protein C[11]
- Chelation therapy, a countermeasure for treating internal radio-isotope contamination
- DPTA, a chelation agent used to eliminate actinides that have been ingested, one of three U.S. Food and Drug Administration (FDA) radioprotectants stockpiled[5]
- Prussian blue/radiogardase, a chelation agent to treat radio-Cesium and thallium consumption, one of three the FDA radioprotectants stockpiled[5]
- Potassium iodide, a prophylactic drug recommended before entering radioiodine environments, one of three FDA radioprotectants stockpiled[5]
- Kojic acid
- Hyaluronan
- Petkau effect
References
- "Onconova Therapeutics presents new data demonstrating radioprotection by Ex-RAD at RRS annual meeting" (Press release). EurekAlert. 2010-09-27. Archived from the original on 2011-03-22. Retrieved 2011-03-22.
- "Recilisib". Adis Insight.
- Ghosh, Sanchita P.; Perkins, Michael W.; Hieber, Kevin; Kulkarni, Shilpa; Kao, Tzu-Cheg; Reddy, E. Premkumar; Reddy, M. V. Ramana; Maniar, Manoj; Seed, Thomas; Kumar, K. Sree (2009). "Radiation Protection by a New Chemical Entity, Ex-Rad: Efficacy and Mechanisms". Radiation Research. 171 (2): 173–9. Bibcode:2009RadR..171..173G. doi:10.1667/RR1367.1. PMID 19267542.
- "Ex-RAD® for Protection from Radiation Injury". Onconova Therapeutics. 2009. Archived from the original on 2011-03-22. Retrieved 2011-03-22.
- "The Drugs That May Never Be Used". Chemical & Engineering News. 90 (26): 23–26. 2012.
- Kouvaris, J. R.; Kouloulias, V. E.; Vlahos, L. J. (2007). "Amifostine: The First Selective-Target and Broad-Spectrum Radioprotector". The Oncologist. 12 (6): 738–47. doi:10.1634/theoncologist.12-6-738. PMID 17602063.
- Reliene, Ramune; Pollard, Julianne M.; Sobol, Zhanna; Trouiller, Benedicte; Gatti, Richard A.; Schiestl, Robert H. (2009). "N-acetyl cysteine protects against ionizing radiation-induced DNA damage but not against cell killing in yeast and mammals". Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 665 (1–2): 37–43. doi:10.1016/j.mrfmmm.2009.02.016. PMID 19427509.
- Mansour, Heba H.; Hafez, Hafez F.; Fahmy, Nadia M.; Hanafi, Nemat (2008). "Protective effect of N-acetylcysteine against radiation induced DNA damage and hepatic toxicity in rats". Biochemical Pharmacology. 75 (3): 773–80. doi:10.1016/j.bcp.2007.09.018. PMID 18028880.
- Demirel, C; Kilçiksiz, S; Ay, OI; Gürgül, S; Ay, ME; Erdal, N (2009). "Effect of N-acetylcysteine on radiation-induced genotoxicity and cytotoxicity in rat bone marrow". Journal of Radiation Research. 50 (1): 43–50. Bibcode:2009JRadR..50...43D. doi:10.1269/jrr.08066. PMID 19218780.
- Demirel, C; Kilciksiz, S; Evirgen-Ayhan, S; Gurgul, S; Erdal, N (2010). "The preventive effect of N-acetylcysteine on radiation-induced dermatitis in a rat model". Journal of the Balkan Union of Oncology. 15 (3): 577–82. PMID 20941831.
- Geiger, Hartmut; Pawar, Snehalata A; Kerschen, Edward J; Nattamai, Kalpana J; Hernandez, Irene; Liang, Hai Po H; Fernández, Jose Á; Cancelas, Jose A; Ryan, Marnie A; Kustikova, Olga; Schambach, Axel; Fu, Qiang; Wang, Junru; Fink, Louis M; Petersen, Karl-Uwe; Zhou, Daohong; Griffin, John H; Baum, Christopher; Weiler, Hartmut; Hauer-Jensen, Martin (2012). "Pharmacological targeting of the thrombomodulin–activated protein C pathway mitigates radiation toxicity". Nature Medicine. 18 (7): 1123–9. doi:10.1038/nm.2813. PMC 3491776. PMID 22729286.