Disiloxane
Disiloxane has the chemical formula Si
2H
6O. It is the simplest known siloxane with hydrogen only R groups. The molecule contains six equivalent Si-H bonds and two equivalent Si-O bonds. Disiloxane exists as a colorless, pungent gas under standard conditions. However, it is generally safe for human use as evidence in its widespread use in cosmetics. It is also commonly known as disilyl ether, disilyl oxide, and perhydrodisiloxane
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Disiloxane | |
| Other names
Disilyl ether Disilyl oxide | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | DS DSE |
| ChEBI | |
| ChemSpider | |
| 1206 | |
| MeSH | Disiloxane |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| H6OSi2 | |
| Molar mass | 78.217 g·mol−1 |
| Appearance | Colorless gas |
| Boiling point | −15.2 °C (4.6 °F; 257.9 K) |
| 0.24 D | |
| Structure | |
| Orthorhombic | |
| Pmm2 | |
| Bent | |
| Hazards | |
| Safety data sheet | See: data page |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Related compounds |
Dimethyl ether |
| Supplementary data page | |
| Refractive index (n), Dielectric constant (εr), etc. | |
Thermodynamic data |
Phase behaviour solid–liquid–gas |
| UV, IR, NMR, MS | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Structure
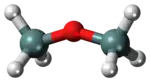
Disiloxane has a simple structure that consists of a siloxane bond (Si-O-Si) and hydrogen R groups.
The structure of disiloxane has been studied by a variety of spectroscopic methods such as electron diffraction, dipole moment, and nuclear magnetic resonance. Commonly, the bond angles of the Si-O-Si bond are studied due to their unusual nature. These bonds are typically exhibit bond angles that are larger than average, around 130 to 160 degrees, larger bond lengths are not uncommon. Typical bond angles with oxygen atoms are around 105 to 125 degrees corresponding to the hybrid s and p orbitals. The abnormally large bond angle has led some scientists to believe that the molecule exhibits a linear geometry while others have collected data indicating that disiloxane exhibits a bent geometry. This disagreement usually depends on the spectroscopic technique used to analyze the compound. The unusual bond angle may be due to unshared electron interactions from oxygen interacting with the d orbitals of silicon. It has been estimated that the two lone pairs of oxygen are each about 50% involved with π bond interactions with silicon’s d orbitals. There is also evidence of π bonding between the p and d orbitals which results in a bond shortening between Si-O which improves the overlap and overall stability of the molecule. Because of this shortening, the Si-O bonds can exhibit some partial double bond behavior.
In addition to studies of bond angles, vibrational analyses have also been done to determine the symmetry elements of disiloxane. IR and Raman spectroscopy have been used to propose a point group of D3d.
Synthesis
Synthesis of disiloxane is typically done by taking a hydrosilane species with a substituent leaving group and reacting it with water to produce silanol. The silanol is then reacted with itself to produce the final disiloxane through dehydrogenative coupling. This is shown in the reactions below:
H3SiX + H2O → H3SiOH + HX (first step)
2 H3SiOH → H3SiOSiH3 + H2O (second step)
Other methods of synthesis involve the use of gold on carbon as a catalyst for the reaction carried out in water as well as InBr3- catalyzed oxidation of hydrosilanes.
Uses
Disiloxanes can be used as sealants for construction, paints, inks, and coatings, cosmetics, mechanical fluids, textile applications, and paper coatings.
Commercial use of disiloxane is common in cosmetics. It is commonly found in products such as sunscreen, moisturizer, hair spray, eye liner, body spray, nail polish, makeup remover, and conditioner. The properties that disiloxane exhibits in these products include fast drying, oil reducing, moisturizing, skin conditioning, and defoaming agent (preventing formation of foam).
Disiloxanes have been approved as teen and child safe. Siloxanes of many kinds are found to be extremely safe for topical use but can be dangerous if ingested.
Variations
The term disiloxane is commonly used to refer to structures that exhibit much more complex R groups than hydrogen. The most common molecule that makes use of this naming is Hexamethyldisiloxane which replaces the hydrogen groups with methyl groups. Other common variations include the use of disiloxanes as bridges and spacers in larger compounds such as polymers.
References
- Sawama, Y.; Masuda, M.; Yasukawa, N.; Nakatani, R.; Nishimura, S.; Shibata, K.; Yamada, T.; Monguchi, Y.; Suzuka, H.; Takagi, Y.; Sajiki, H. The Journal of Organic Chemistry2016, 81, 4190-4195.
- Disiloxane https://pubchem.ncbi.nlm.nih.gov/compound/Disiloxane (accessed Mar 23, 2018).
- Lassen, C.; Hansen, C.; Mikkelsen, S.; Maag, J. Siloxanes-Consumption, Toxicity, and Alternatives; Danish Ministry of the Environment, 2018.
- Almennigen, A.; Bastiansen, O.; Ewing, V.; Hedberg, K.; Tretteberg, M. ACTA Chemica Scandinavica 2018, 17, 2455-2460.
- Lord, R.; Robinson, D.; Schumb, W. Journal of the American Chemical Society 1956, 78, 1327-1332.
- Barrow, M.; Ebsworth, E.; Harding, M. Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry 1979, 35, 2093-2099.
- Varma, R.; MacDiarmid, A.; Miller, J. Inorganic Chemistry 1964, 3, 1754-1757.
- BOCK, H.; MOLLERE, P.; BECKER, G.; FRITZ, G. Chemischer Informationsdienst 1974, 5, 113-125.
- Disiloxane | H6OSi2 | ChemSpider http://www.chemspider.com/Chemical-Structure.109921.html (accessed Mar 23, 2018).
