Dipicolylamine
Dipicolylamine is an organic compound with the formula HN(CH2C5H4N)2. It is a yellow liquid that is soluble in polar organic solvents. The molecule is a secondary amine with two picolyl substituents. The compound is a common tridentate ligand in coordination chemistry.[1][2]
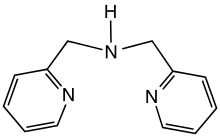 | |
| Names | |
|---|---|
| Other names
Di-(2-picolyl)amine, DPA | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.014.788 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H13N3 | |
| Molar mass | 199.25 |
| Appearance | yellow liquid |
| Density | 1.107 g/cm3 |
| Boiling point | 139 to 141 °C at 1 mmHg |
| low | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The compound can be prepared by many methods, alkylation of picolinylamine with picolinyl chloride, deamination of picolinylamine, and reductive amination of picolinyl amine and pyridine-2-carboxaldehyde. It is commonly used to bind to bacteria in purifying mixtures that require separation.
Related compounds
References
- Sakamoto, Takashi; Ojida, Akio; Hamachi, Itaru"Molecular recognition, fluorescence sensing, and biological assay of phosphate anion derivatives using artificial Zn(II)-Dpa complexes" Chemical Communications 2009, pp.141-152. doi:10.1039/B812374H
- Huy Tien Ngo, Xuejian Liu, Katrina A. Jolliffe "Anion recognition and sensing with Zn(II)–dipicolylamine complexes" Chem. Soc. Rev., 2012,41, 4928-4965. doi:10.1039/C2CS35087D
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.