Diphenyl carbonate
Diphenyl carbonate is the organic compound with the formula (C6H5O)2CO. It is classified as an acyclic carbonate ester. It is a colorless solid. It is both a monomer in combination with bisphenol A in the production of polycarbonate polymers[2][3] and a product of the decomposition of polycarbonates.[4]
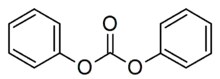 | |
 | |
| Names | |
|---|---|
| IUPAC name
Diphenyl carbonate | |
| Other names
Phenyl carbonate | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.002.733 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C13H10O3 | |
| Molar mass | 214.216 g/mol |
| Density | 1.1215 g/cm3 at 87 °C |
| Melting point | 83 °C (181 °F; 356 K) |
| Boiling point | 306 °C (583 °F; 579 K) |
| insoluble | |
| Solubility | soluble in ethanol, diethyl ether, carbon tetrachloride, acetic acid[1] |
| Hazards | |
| GHS pictograms |   |
| GHS Signal word | Warning |
| H302, H400, H410, H411 | |
| P264, P270, P273, P301+312, P330, P391, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Production
World production capacity of diphenyl carbonate was 254,000 tonnes in 2002, and phosgenation of phenol is the most significant route.[5] Phosgenation of phenol can proceed under various conditions. The net reaction is as follows:
- 2 PhOH + COCl2 → PhOCO2Ph + 2 HCl
The use of phosgene can be avoided by the oxidative carbonylation of phenol with carbon monoxide:[2]
- 2 PhOH + CO + [O] → PhOCO2Ph + H2O
Dimethyl carbonate can also be transesterified with phenol:
- CH3OCO2CH3 + 2 PhOH → PhOCO2Ph + 2 MeOH
The kinetics and thermodynamics of this reaction are not favorable. For example, at higher temperatures, dimethyl carbonate undesirably methylates phenol to give anisole.[2]
Applications
Polycarbonates can be prepared by transesterifying diphenyl carbonate with bisphenol A. Phenol is a co-product. These polycarbonates may be recycled by reversing the process: transesterifying the polycarbonate with phenol to yield diphenyl carbonate and bisphenol A.[2]
References
- Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, FL: CRC Press, pp. 3–238, ISBN 0-8493-0594-2
- Hans-Josef Buysch. "Carbonic Esters". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a05_197/full.
- Wittcoff, Harold; Reuben, B. G.; Plotkin, Jeffrey S. (2004), Industrial Organic Chemicals, Wiley-IEEE, p. 278, ISBN 978-0-471-44385-8, retrieved 2009-07-20
- ASM International (2003), Characterization and Failure Analysis of Plastics, ASM International, p. 369, ISBN 978-0-87170-789-5, retrieved 2009-07-20
- "Diphenyl Carbonate" (PDF). IPSC Inchem. Retrieved 2012-08-01.