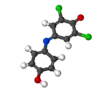
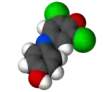
Dichlorophenolindophenol
2,6-Dichlorophenolindophenol (DCPIP, DCIP or DPIP) is a chemical compound used as a redox dye. When oxidized, DCPIP is blue with a maximal absorption at 600 nm; when reduced, DCPIP is colorless.
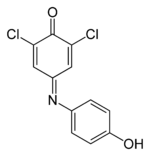 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
2,6-Dichloro-4-[(4-hydroxyphenyl)imino]cyclohexa-2,5-dien-1-one | |||
| Other names
Dichloroindophenol (); 2,6-Dichlorophenolindophenol; | |||
| Identifiers | |||
3D model (JSmol) |
|||
| Abbreviations | DCPIP, DCIP, DPIP | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.012.254 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C12H7Cl2NO2 | |||
| Molar mass | 268.09 g·mol−1 | ||
| Hazards | |||
| GHS pictograms |  | ||
| GHS Signal word | Warning | ||
| H302, H315, H319, H335 | |||
| P261, P264, P270, P271, P280, P301+312, P302+352, P304+340, P305+351+338, P312, P321, P330, P332+313, P337+313, P362, P403+233, P405, P501 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
DCPIP can be used to measure the rate of photosynthesis. It is part of the Hill reagents family. When exposed to light in a photosynthetic system, the dye is decolorised by chemical reduction. DCPIP has a higher affinity for electrons than ferredoxin and the photosynthetic electron transport chain can reduce DCPIP as a substitute for NADP+, that is normally the final electron carrier in photosynthesis. As DCPIP is reduced and becomes colorless, the resultant increase in light transmittance can be measured using a spectrophotometer.
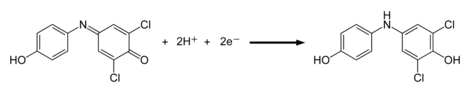
DCPIP can also be used as an indicator for vitamin C.[1] If vitamin C, which is a good reducing agent, is present, the blue dye, which turns pink in acid conditions, is reduced to a colorless compound by ascorbic acid. This reaction is a redox reaction: vitamin C (ascorbic acid) is oxidized to dehydroascorbic acid, and DCPIP is reduced to the colorless compound DCPIPH2
- DCPIP (blue) + H+ → DCPIPH (pink)
- DCPIPH (pink) + vitamin C → DCPIPH2 (colorless)
In this titration, when all the ascorbic acid in the solution has been used up, there will not be any electrons available to reduce the DCPIPH and the solution remains pink due to the DCPIPH. The end point is a pink color that persists for 10 seconds or more, if there is not enough ascorbic acid to reduce all of the DCPIPH. Pharmacological experiments suggest that DCPIP may serve as a pro-oxidant chemotherapeutic targeting human cancer cells in an animal model of human melanoma; DCPIP-induced cancer cell death occurs by depletion of intracellular glutathione and upregulation of oxidative stress.[2]
References
- VanderJagt DJ, Garry PJ, Hunt WC (June 1986). "Ascorbate in plasma as measured by liquid chromatography and by dichlorophenolindophenol colorimetry". Clin. Chem. 32 (6): 1004–6. PMID 3708799.
- Cabello CM, Bair WB, Bause AS, Wondrak GT (August 2009). "Antimelanoma activity of the redox dye DCPIP (2,6-dichlorophenolindophenol) is antagonized by NQO1". Biochem. Pharmacol. 78 (4): 344–54. doi:10.1016/j.bcp.2009.04.016. PMC 2742658. PMID 19394313.