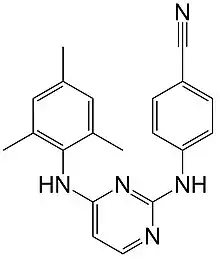
Dapivirine
Dapivirine is a non-nucleoside reverse transcriptase inhibitor developed at Janssen Therapeutics (formerly Tibotec Therapeutics).[1] The International Partnership for Microbicides has held exclusive worldwide rights to dapivirine since 2014,[2] building upon a 2004 royalty-free license to develop dapivirine-based microbicides for women in resource-poor countries.[3]
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C20H19N5 |
| Molar mass | 329.398 |
| 3D model (JSmol) | |
| |
| |
A monthly intravaginal ring containing dapivirine has been developed as a way of preventing infection by human immunodeficiency virus in women. Two phase 3 clinical trials of intravaginal dapivirine rings for HIV prevention were completed in 2015 and results were announced at the 2016 Conference on Retroviruses and Opportunistic Infections. The ASPIRE Study (MTN-020) reported a 27% reduction in HIV-1 acquisition (95% CI 12-57%, p=0.007), with a trend toward greater protection in women over age 21 and no significant protection for women under age 21.[4] The Ring Study (IPM-027) reported a 31% reduction in HIV acquisition (95% CI 0.9-51.5%, p=0.040) also with a trend toward greater efficacy in women over age 21.[5] In both trials, more than 80% of returned rings showed signs of drug depletion indicating at least some use, and more than 80% of blood samples from participants in the active arm had levels of dapivirine consistent at least 8 hours of continuous use preceding the blood test. Neither trial could evaluate whether the product was used consistently between study visits.
As of December 2019, it became the first of its kind to be submitted for regulatory approval.[6][7] The ring is currently under review by the European Medicines Agency with an opinion expected in 2020. Further regulatory submissions are planned to the US Food and Drug Administration, the South African Health Products Regulatory Authority, and other regulators in Africa where women face the highest risk for HIV.[8]
External Links
References
- "Johnson & Johnson to Acquire Tibotec-Virco (NYSE:JNJ)". www.investor.jnj.com. Retrieved 2016-04-13.
- hseltzer. "IPM Receives Worldwide Rights to HIV Prevention Medicine". www.ipmglobal.org. Retrieved 2016-04-13.
- ipm-admin. "Dapivirine (TMC120)". www.ipmglobal.org. Retrieved 2016-04-13.
- Jared M. Baeten. "A Phase III Trial of the Dapivirine Vaginal Ring for HIV-1 Prevention in Women". Retrieved 2016-04-13.
- Annalene Nel. "Safety and Efficacy of Dapivirine Vaginal Ring for HIV-1 Prevention in African Women". Retrieved 2016-04-13.
- PrEWatch.org. "Nextgen-Dapivirine Vaginal Ring". Retrieved 2020-05-26.
- ipmglobal.org. "Dapivirine Ring". Retrieved 2020-05-26.
- ipmglobal.org. "Dapivirine Ring". Retrieved 2020-05-26.