Chlorosulfonyl isocyanate
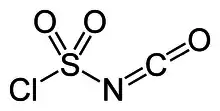
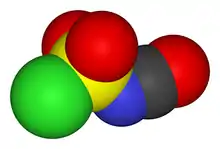
Chlorosulfonyl isocyanate is the chemical compound ClSO2NCO, known as CSI. This compound is a versatile reagent in organic synthesis.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Chlorosulfonyl isocyanate | |
| Other names
N-Carbonylsulfamyl chloride Chloropyrosulfonyl isocyanate Sulfuryl chloride isocyanate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.013.378 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| CNClO3S | |
| Molar mass | 141.53 g/mol |
| Appearance | colorless liquid |
| Density | 1.626 g/cm3 |
| Melting point | −44 °C (−47 °F; 229 K) |
| Boiling point | 107 °C (225 °F; 380 K) |
| decomposition | |
| Solubility in other solvents | Chlorocarbons MeCN |
Refractive index (nD) |
1.447 |
| Structure | |
| tetrahedral at S | |
| Hazards | |
| Main hazards | toxic, corrosive, flammable, reacts violently with water |
| Safety data sheet | "External MSDS" |
| GHS pictograms |     |
| GHS Signal word | Danger |
| H302, H312, H314, H330, H332, H334 | |
| P260, P261, P264, P270, P271, P280, P284, P285, P301+312, P301+330+331, P302+352, P303+361+353, P304+312, P304+340, P304+341, P305+351+338, P310, P312, P320, P321, P322, P330, P342+311, P363, P403+233 | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Related compounds |
Thionyl chloride Cyanogen bromide Phosphoryl chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation, structure, handling
CSI is prepared by treating cyanogen chloride with sulfur trioxide, the product being distilled directly from the reaction mixture.[1]
- SO3 + ClCN → ClSO2NCO
In this transformation, both the carbon and the nitrogen termini of CN are functionalized.
The structure of CSI is represented as ClS(O)2-N=C=O. It consists of two electron-withdrawing components, the chlorosulfonyl group (SO2Cl) and the isocyanate group (-N=C=O). Because of its resulting electrophilicity, the use of CSI in chemical synthesis requires relatively inert solvents such as chlorocarbons, acetonitrile, and ethers.[2]
Uses
The molecule has two electrophilic sites, the carbon and the S(VI) center.[3]
CSI has been employed for the preparation of β-lactams,[4] some of which are medicinally important. Thus, alkenes undergo a [2+2]-cycloaddition to give the sulfonamide. The SO2Cl group can be removed simply by hydrolysis, leaving the secondary amide.[5] Other reactions of CSI:
- Cycloaddition to alkynes to give 1,2,3-oxathiazine-2,2-dioxide-6-chlorides.
- Conversion of primary alcohols to carbamates.[6]
- Conversion of carboxylic acids and the acid chlorides into nitriles.
- Preparation of N,N-disubstituted sulfamides, R2NSO2NH2
- Preparation of Burgess reagent
Safety considerations
CSI is toxic, corrosive and reacts violently with water. Like hydrofluoric acid, it cannot be stored in glassware, requiring instead polyethylene bottles.
References
- Graf, R. "Chlorosulfonyl Isocyanate" Organic Syntheses, Collected Volume 5, pages 226ff.
- Miller, M. J.; Ghosh, M.; Guzzo, P. R.; Vogt, P. F.; Hu, J.; Filzen, G. F.; Geyer, A. G. "Chlorosulfonyl Isocyanate" in "Encyclopedia of Reagents for Organic Synthesis" 2005 John Wiley & Sons: New York.
- D. N. Dhar, K. S. K. Murthy "Recent Advances in the Chemistry of Chlorosulfonyl Isocyanate" Synthesis 1986; pages 437-449.
- Kaur, Rajneesh; Singh, Raman; Kumar, Antresh; Kaur, Satvinder; Priyadarshi, Nitesh; Singhal, Nitin Kumar; Singh, Kuldeep (June 2020). "1,2,3-Triazole β-lactam conjugates as antimicrobial agents". Heliyon. 6 (6): e04241. doi:10.1016/j.heliyon.2020.e04241. PMC 7327255. PMID 32637684.
- Cremlyn, R. J. “An Introduction to Organosulfur Chemistry” John Wiley and Sons: Chichester (1996). ISBN 0-471-95512-4
- Burgess, E. M.; Penton, Jr., H. R.; Taylor, E. A.; Williams, W. M. "Conversion of Primary Alcohols to Urethanes via the Inner Salt of Triethylammonium Hydroxide: Methyl (Carboxylsulfamoyl) Triethylammonium Hydroxide Methyl n-Hexylcarbamate" Organic Syntheses, Coll. Vol. 6, p.788
