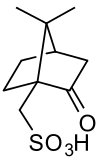
Camphorsulfonic acid
Camphorsulfonic acid, sometimes abbreviated CSA or 10-CSA is an organosulfur compound. Like typical sulfonic acids, it is a relatively strong acid that is a colorless solid at room temperature and is soluble in water and a wide variety of organic substances.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
(7,7-dimethyl-2-oxobicyclo[2.2.1]heptan-1-yl)methanesulfonic acid | |||
| Other names
Reychler's acid; 2-Oxobornane-10-sulfonic acid | |||
| Identifiers | |||
| |||
3D model (JSmol) |
|||
| 2216194 | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.025.024 | ||
| EC Number |
| ||
| MeSH | 10-Camphorsulfonic+acid | ||
PubChem CID |
|||
| UNII |
| ||
| UN number | 1759 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C10H16O4S | |||
| Molar mass | 232.29 g·mol−1 | ||
| Melting point | 195 °C (decomposes) | ||
| Acidity (pKa) | 1.2 | ||
| Hazards | |||
| Safety data sheet | External MSDS | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
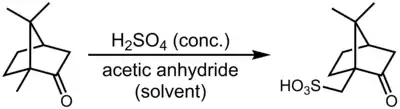
This compound is commercially available. It can be prepared by sulfonation of camphor with sulfuric acid and acetic anhydride:[1]
Although this reaction appears to be a sulfonation of an unactivated methyl group, the actual mechanism is believed to involve a retro-semipinacol rearrangement, deprotonation next to the tertiary carbocation to form an alkene, sulfonation of the alkene intermediate, and finally, semipinacol rearrangement to re-establish the ketone function.[2]
In organic synthesis, CSA and its derivatives can be used as resolving agents for chiral amines and other cations.[3][4] The synthesis of osanetant was an example of this. 3-bromocamphor-8-sulfonic acid was used in the synthesis of enantiopure devazepide.[5] Recently Camphorsulfonic acid is also being used for the synthesis of quinolines[6]
Camphorsulfonic acid is used in some pharmaceutical formulations, where is it referred to as camsilate or camsylate, including trimetaphan camsilate and lanabecestat camsylate.
References
- Paul D. Bartlett and L. H. Knox (1973). "D,L-10-Camphorsulfonic acid (Reychler's Acid)". Organic Syntheses.; Collective Volume, 5, p. 194
- 1955-, Brückner, Reinhard (2002). Advanced organic chemistry : reaction mechanisms. San Diego: Harcourt/Academic Press. ISBN 9780080498805. OCLC 269472848.CS1 maint: numeric names: authors list (link)
- D. Clark, Robin; R. Kern, John; J. Kurz, Lilia; T. Nelson, Janis (1990). "Preparation of Enatiomerically Pure Decahydro-6H-isoquino[2,1-g][1,6]naphthyridines Utilizing the Openshaw-Whittaker Hexahydrobenzo[a]quinolizinone Resolution". Heterocycles. 31 (2): 353. doi:10.3987/COM-89-5250.
- André B. Charette "3-Bromocamphor-8-sulfonic Acid" Encyclopedia of Reagents for Organic Synthesis 2001, John Wiley & Sons. doi:10.1002/047084289X.rb283
- Reider, Paul J.; Davis, Paul; Hughes, David L.; Grabowski, Edward J. J. (1987). "Crystallization-induced asymmetric transformation: Stereospecific synthesis of a potent peripheral CCK antagonist". J. Org. Chem. 52 (5): 955–957. doi:10.1021/jo00381a052.
- Chandra, Devesh; Dhiman, Ankit K; Kumar, Rakesh; Sharma, Upendra (2019). "Microwave‐Assisted Metal‐Free Rapid Synthesis of C4‐Arylated Quinolines via Povarov Type Multicomponent Reactiont". Eur. J. Org. Chem. 2019 (16): 2753–2758. doi:10.1002/ejoc.201900325.