Bacterial anaerobic corrosion
Bacterial anaerobic corrosion is a bacterially-induced oxidation of metals.
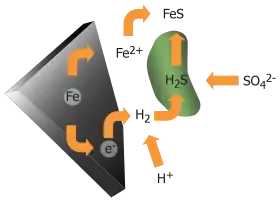
In a humid environment and anoxic conditions the corrosion of metals occurs as a result of a redox reaction that generates molecular hydrogen from hydrogen ions, requiring bacteria, unlike anaerobic corrosion that occurs spontaneously.
A base metal, such as iron (Fe) goes into aqueous solution as positively charged cation, Fe2+. As the metal is oxidized under anaerobic condition by the protons of water, H+ ions are reduced to form molecular H2. This can be written in the following ways under acidic and neutral conditions respectively:
- Fe + 2 H+ → Fe2+ + H2
- Fe + 2 H2O → Fe(OH)2 + H2
Usually, a thin film of molecular hydrogen forms on the metal. Sulfate-reducing bacteria, oxidize the molecular hydrogen to produce hydrogen sulfide ions (HS−) and water:
- 4 H2 + SO42− → HS− + 3 H2O + OH−
The iron ions partly precipitate to form iron (II) sulfide. A reaction with water also occurs, producing iron hydroxide.
- Fe2+ + HS− → FeS + H+
- 3 Fe2+ + 6 H2O → 3 Fe(OH)2 + 6 H+
The net equation comes to:
- 4 Fe + SO42− + H+ + 3 H2O → FeS + 3 Fe(OH)2 + OH−
This form of corrosion by sulfate-reducing bacteria can, in this way, be far more harmful than anaerobic corrosion.