Apoatropine
Apoatropine is a member of class of tropane alkaloids. Apoatropine can be found in plants of family Solanaceae. It is a bitter crystalline alkaloid. Examples of related tropane alkaloids include atropine, hyoscyamine, and hyoscine. Though apoatropine is found in various plants, it can also be prepared by the dehydration of atropine using nitric acid . Apoatropine is used as a pigment.
 | |
| Names | |
|---|---|
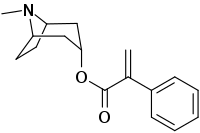
| IUPAC name
(8-Methyl-8-azabicyclo[3.2.1]octan-3-yl) 2-phenylprop-2-enoate | |
| Other names
Apoatropine Hydrochloride, Atropamin Hydrochloride, Atropyltropeine Hydrochloride, Apoascyamine, and Atropane. | |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.007.188 |
PubChem CID |
|
| |
| Properties | |
| C17H21NO2 | |
| Molar mass | 271.360 g·mol−1 |
| Appearance | White or off whiteish and crystalline |
| Melting point | >236 °C (HCl salt, decomposes)[2] |
| Soluble in water, alcohol, and ether | |
| Hazards | |
| Main hazards | Considered poisonous |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- Pubchem. "Apoatropine". nih.gov.
- "Apoatropine Hydrochloride". Santa Cruz Biotechnology.
- Krantz, J. C.; Forrest, J. W.; Heisse, C. K. (1954). "Contribution to the Pharmacology of Apoatropine and Its Methyl Bromide". Experimental Biology and Medicine. 86 (3): 511–512. doi:10.3181/00379727-86-21150. ISSN 1535-3702.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.