Antipain
Antipain is an oligopeptide that is isolated from actinomycetes and used in biochemical research as a protease inhibitor of trypsin and papain.[1] It was discovered in 1972 and was the first natural peptide found that contained an ureylene group.[2]
 | |
| Names | |
|---|---|
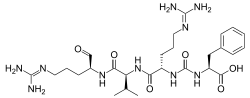
| IUPAC name
N2-{[(1S)-1-carboxy-2-phenylethyl]carbamoyl}-N5-(diaminomethylidene)-L-ornithyl-N-{(2S)-5-[(diaminomethylidene)amino]-1-oxopentan-2-yl}-L-valinamide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C27H44N10O6 | |
| Molar mass | 604.713 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It has been crystallised in complexes with carboxypeptidase, which is obtained from wheat,[3] and Leishmania major oligopeptidase B.[4] In both cases, the backbone carbonyl of the terminal arginine of antipain forms a covalent bond to the active site serine in the protease.
Antiretroviral and Protease inhibitors
There are several Serine proteases, which are enzymes that cleave the protein bond, in the human genome. The abnormal functioning of these proteases can lead to the development of cancerous tumors.[5][6] Protease inhibitors or antipain are enzymes that are used to regulate their performance.
The antiretroviral drug Nelfinavir is one example of an antipain. It was classified as an antipain after a study published by Ovid that investigated the in vitro effect of Nelfinavir using proteolytic foot printing and found that it selectively inhibited HER2- positive, a growth factor in breast cancer.[7]
Protease inhibitors and DRUG neurons
Protease-activated receptors (PARs) are a unique class of G protein-coupled receptors activated by proteolytic cleavage of the receptor N terminus.[8] PARs are activated by some Serine Proteases and are important for the physiological, psychological,[9] and pathological functions of the human body.
During the study, an antipain analogue Y was developed and studied. It was shown to have properties as a protease inhibitors but it had a low IC50 than a antipain. Antipain analogue Y was able to suppress Trypsin, which inhibits the secretion of an excitatory neuropeptide that leads to inflammation and other disorders. {{Citation needed}}
References
- Suda, H; Aoyagi, T; Hamada, M; Takeuchi, T; Umezawa, H (1972). "Antipain, a new protease inhibitor isolated from actinomycetes". The Journal of Antibiotics. 25 (4): 263–6. doi:10.7164/antibiotics.25.263. PMID 4559651.
- Umezawa, S; Tatsuta, K; Fujimoto, K; Tsuchiya, T; Umezawa, H (1972). "Structure of antipain, a new Sakaguchi-positive product of streptomyces". The Journal of Antibiotics. 25 (4): 267–70. doi:10.7164/antibiotics.25.267. PMID 5052959.
- PDB ENTRY 1bcr Bullock, TL; Breddam, K; Remington, SJ (1996). "Peptide aldehyde complexes with wheat serine carboxypeptidase II: implications for the catalytic mechanism and substrate specificity". J. Mol. Biol. 255 (5): 714–25. doi:10.1006/jmbi.1996.0058. PMID 8636973.
- PDB ENTRY 2xe4 McLuskey, K; Paterson, NG; Bland, ND; Isaacs, NW; Mottram, JC (2010). "Crystal Structure of Leishmania Major Oligopeptidase B Gives Insight Into the Enzymatic Properties of a Trypanosomatid Virulence Factor". J. Biol. Chem. 285 (50): 39249–59. doi:10.1074/jbc.M110.156679. PMC 2998157. PMID 20926390.
- Quesada, V.; Ordonez, G. R.; Sanchez, L. M.; Puente, X. S.; Lopez-Otin, C. (1 January 2009). "The Degradome database: mammalian proteases and diseases of proteolysis". Nucleic Acids Research. 37 (Database): D239–D243. doi:10.1093/nar/gkn570. PMC 2686449. PMID 18776217.
- Mariño, Guillermo; Urı́a, José A.; Puente, Xose S.; Quesada, Vı́ctor; Bordallo, Javier; López-Otı́n, Carlos (7 February 2003). "Human Autophagins, a Family of Cysteine Proteinases Potentially Implicated in Cell Degradation by Autophagy". Journal of Biological Chemistry. 278 (6): 3671–3678. doi:10.1074/jbc.M208247200. PMID 12446702.
- Shim, Joong Sup; Rao, Rajini; Beebe, Kristin; Neckers, Len; Han, Inkyu; Nahta, Rita; Liu, Jun O. (17 October 2012). "Selective Inhibition of HER2-Positive Breast Cancer Cells by the HIV Protease Inhibitor Nelfinavir". JNCI: Journal of the National Cancer Institute. 104 (20): 1576–1590. doi:10.1093/jnci/djs396. PMC 3472971. PMID 23042933.
- Nakae, Koichi; Kojima, Fukiko; Sawa, Ryuichi; Kubota, Yumiko; Igarashi, Masayuki; Kinoshita, Naoko; Adachi, Hayamitu; Nishimura, Yoshio; Akamatsu, Yuzuru (January 2010). "Antipain Y, a new antipain analog that inhibits neurotransmitter release from rat dorsal root ganglion neurons". The Journal of Antibiotics. 63 (1): 41–44. doi:10.1038/ja.2009.109. ISSN 1881-1469. PMID 19911027.
- Nakae, Koichi; Saito, Kiyoko; Iino, Takashi; Yamamoto, Noriyuki; Wakabayashi, Mayo; Yoshikawa, Satoru; Matsushima, Satoshi; Miyashita, Hiroshi; Sugimoto, Hiromi; Kiba, Atushi; Gupta, Jung (December 2005). "A Prostacyclin Receptor Antagonist Inhibits the Sensitized Release of Substance P from Rat Sensory Neurons". Journal of Pharmacology and Experimental Therapeutics. 315 (3): 1136–1142. doi:10.1124/jpet.105.091967. PMID 16109742. S2CID 14841421.