Alpine borane
Alpine borane is the commercial name for an organoboron compound that is used in organic synthesis. It is a colorless liquid, although it is usually encountered as a solution.
 | |
 | |
| Names | |
|---|---|
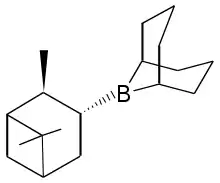
| IUPAC name
9-(2,6,6-Trimethylbicyclo[3.1.1]hept-3-yl)-9-bora-bicyclo[3.3.1]nonane | |
| Other names
Alpine-Borane; B-Isopinocampheyl-9-borabicyclo[3.3.1]nonane; B-3-Pinanyl-9-borabicyclo[3.3.1]nonane | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.157.575 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C18H31B | |
| Molar mass | 258.26 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.947 g/mL |
| Boiling point | > 55 °C (131 °F; 328 K) |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Danger |
| H250 | |
| P210, P222, P280, P302+334, P370+378, P422 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation and reactions
This reagent is generated by treating 9-BBN with α-pinene.[2]
This sterically crowded chiral trialkylborane can stereoselectively reduce aldehydes in what is known as the Midland Alpine Borane Reduction, or simply the Midland Reduction:[3]
- C8H12B-pinanyl + RCDO → C8H12BOCHDR + (+)-d-pinene
Hydrolysis of the resulting borinic ester affords the alcohol:
- C8H12BOCHDR + H2O → C8H12BOH + HOCHDR
It is also effective for the stereoselective reduction of certain acetylenic ketones.[4]
The reaction is proposed to involve formatioin of an adduct by coordination of the carbonyl oxygen to boron. Intramolecular hydride transfer from the pinane substituent to the carbonyl carbon ensues.[2]
Related reagents
A range of alkyl-substituted borane are specialty reagents in organic synthesis. Two such reagents that are closely related to Alpine borane are 9-BBN and diisopinocampheylborane.
References
- R-Alpine-Borane and S-Alpine-Borane at Sigma-Aldrich
- M. M. Midland (1989). "Asymmetric reductions with organoborane reagents". Chem. Rev. 89 (7): 1553–1561. doi:10.1021/cr00097a010.
- M. Mark Midland "B-3-Pinanyl-9-borabicyclo[3.3.1]nonane" in Encyclopedia of Reagents for Organic Synthesis 2001 John Wiley, New York.doi:10.1002/047084289X.rp173. Article Online Posting Date: April 15, 2001
- Midland, M. Mark; Graham, Richard S. (1985). "Asymmetric Reduction of α,β-Acetylenic Ketones with B-3-Pinanyl-9-Borabicyclo[3.3.1]nonane: (R)-(+)-1-Octyln-3-ol". 63: 57. doi:10.15227/orgsyn.063.0057. Cite journal requires
|journal=(help)