Alpha-isomethyl ionone
Alpha-isomethyl ionone is a synthetically made and naturally occurring organic compound found in Brewer's yeasts or the species known as Saccharomyces cerevisiae.[1] The compound is an isomer of methyl ionone. Alpha-isomethyl ionone can be colorless or pale-straw coloured liquid.[2] Its primary scent is flowery and secondary scent is violet. It may also have a woody or orris-like scent.[3] and is often used in flavouring and cosmetic industries for example, aftershave lotions, bath products, hair care products, moisturizers, perfumes, shampoos and skin care products.[4] It is also an ingredient used in Channel No. 5,[5] and other branded products such as Fidji by Guy Laroche.[6] Perfume fragrances that alpha-isomethyl ionone is used in are for example, amber, chypre, violet, mimosa, reseda, iris, orris, cyclamen, chypre, berries, woody notes, ylang-ylang, leather, orange, nut, pistachio, muscatel, and tobacco.[6]
 | |
 | |
| Names | |
|---|---|
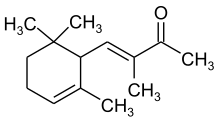
| IUPAC name
(3E)-3-methyl-4-(2,6,6-trimethylcyclohex-2-en-1-yl)but-3-en-2-one | |
| Other names | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ECHA InfoCard | 100.004.407 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C14H22O | |
| Molar mass | 206.3239 |
| Appearance | liquid |
| Density | 0.93 gcm −3 (20 ° C) |
| Boiling point | 93 °C (199 °F; 366 K) (3.1 mmHg) |
| 0.064 g/L | |
| Hazards | |
| Main hazards | irritant, environmental hazard |
| GHS pictograms |  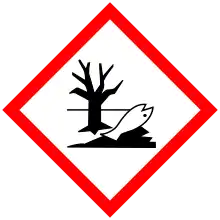 |
| GHS Signal word | Warning |
| H315, H317, H319, H411, H412 | |
| P261, P264, P272, P273, P280, P302+352, P305+351+338, P321, P332+313, P333+313, P337+313, P362, P363, P391, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Alpha-isomethyl ionone can be refined to Cetone alpha[7] which is also used as a fragrance compound. The difference between alpha-isomethyl ionone and cetone alpha is that cetone alpha has a stronger and more intense scent.[3]
Properties

Alpha-isomethyl ionone consists of terpenes with three consecutive isoprene units. They are extremely weak base. It's pKa value (strongest acidic) is 19.7 and it's pKa vlaue (strongest basic) is -4.8.[8] The percentage of alpha-isomethyl ionone used in perfumes are approximately ranging from 0.1% to 11.9%, with an average of 1.1%. For example, in It is usually used in conjunction with hydroxycitronellal, woody notes, copaiba, N-methyl Ionone, Ionone, or Vetiver.[6]
Synthesis
The synthesis of alpha-isomethyl ionone involves a cross- aldol condensation of citral with methyl ethyl ketone[9] A high temperature and strong alkali is used. The ratio between the n-form and iso-form is controlled in order to obtain methyl pseudo-ionone and allow ring formation to occur. Iso-forms is then synthesized consequently.[10]
References
- "alpha-Isomethyl-ionone (YMDB01634)". YMDB. Retrieved 29 May 2020.
- "Alpha-Isomethyl Ionone". Cosmetic Info. Retrieved 29 May 2020.
- "Frangrance Demo Formula". TGSC Information System. Retrieved 24 June 2020.
- "Alfa-Isomethyl ionone". Chemo Technique Diagnostics. Retrieved 29 May 2020.
- Kennedy, James. "Visual Ingredients Chanel No. 5" (PDF). James Kennedy Monash. Retrieved 29 May 2020.
- "Alpha Isomethyl Ionone". Perfumes World. Retrieved 9 July 2020.
- "ChemBK". Cetone alpha. Retrieved 24 June 2020.
- "3-Methyl-4-(2,6,6-trimethyl-2-cyclohexen-1-yl)-3-buten-2-one". Foodb. Retrieved 9 July 2020.
- Burdock, George A. (29 July 2014). Encyclopedia of Food and Colour additives. ISBN 9781498711081. Retrieved 13 July 2020.
- "ALPHA-ISO-METHYLIONONE". Chemical Book. Retrieved 24 June 2020.