Aliquat 336
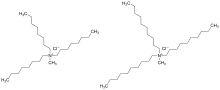
Aliquat 336 (Starks' catalyst) is a quaternary ammonium salt used as a phase transfer catalyst and metal extraction reagent. It contains a mixture of C8 (octyl) and C10 (decyl) chains with C8 predominating. It is an ionic liquid.[1]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
N-Methyl-N,N,N-trioctylammonium chloride | |
| Other names
Starks' catalyst; Tricaprylmethylammonium chloride, Methyltrioctylammonium chloride | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.023.542 |
PubChem CID |
|
| RTECS number |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C25H54ClN | |
| Molar mass | 404.16 g·mol−1 |
| Appearance | Colorless viscous liquid |
| Density | 0.884 g/cm3 |
| Melting point | −20 °C (−4 °F; 253 K) |
| Boiling point | 225 °C (437 °F; 498 K) |
| Viscosity | 1500 mPa·s at 30 °C |
| Hazards | |
| Main hazards | Toxic (USA) |
| Safety data sheet | External MSDS |
| GHS pictograms | 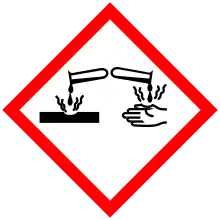   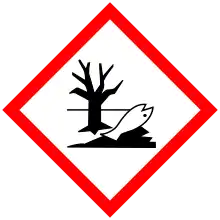 |
| GHS Signal word | Danger |
| H301, H315, H318, H319, H400, H410 | |
| P264, P270, P273, P280, P301+310, P302+352, P305+351+338, P310, P321, P330, P332+313, P337+313, P362, P391, P405, P501 | |
| Flash point | 113 °C (235 °F; 386 K) (closed cup) |
| Related compounds | |
Related |
Aliquat 100, Aliquat 134, Aliquat 175, Aliquat HTA-1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Applications
Organic Chemistry
Aliquat 336 is used as a phase transfer catalyst,[2] including in the catalytic oxidation of cyclohexene to 1,6-hexanedioic acid.[3] This reaction is more environmentally friendly. It is an example of green chemistry, compared with the traditional method of oxidizing cyclohexanol or cyclohexanone with nitric acid or potassium permanganate, which produce hazardous wastes.[4]
Aliquat 336 was used in the total synthesis of manzamine A by Darren Dixon in an early step to the electrophile.[5]
Solvent extraction of metals
Aliquat 336 has been used for the extraction of metals, it does so by acting as a liquid anion exchanger. It is often used while diluted in hydrocarbon solvents such as aromatic kerosene. It is possible to use it in aliphatic kerosene but in such solvents often a phase modifier (typically a long chain alcohol) must be added to prevent the formation of third phase.
References
- Litaiem, Yousra; Dhahbi, Mahmoud (2015). "Physicochemical Properties of an Hydrophobic Ionic Liquid (Aliquat 336) in a Polar Protic Solvent (Formamide) at Different Temperatures". Journal of Dispersion Science and Technology. 36 (5): 641. doi:10.1080/01932691.2013.862170.
- C. M. Starks (1971). "Phase-transfer catalysis. I. Heterogeneous reactions involving anion transfer by quaternary ammonium and phosphonium salts". J. Am. Chem. Soc. 93: 195–199. doi:10.1021/ja00730a033.
- S. M. Reed; J. E. Hutchison (2000). "An Environmentally Benign Synthesis of Adipic Acid". J. Chem. Educ. 77 (12): 1627–8. doi:10.1021/ed077p1627.
- Ameta, Suresh C; Ameta, Rakshit (2013-09-11). Green Chemistry: Fundamentals and Applications. ISBN 9781466578265.
- Jakubec, Pavol; Hawkins, Alison; Felzmann, Wolfgang; Dixon, Darren J. (2012). "Total Synthesis of Manzamine A and Related Alkaloids". Journal of the American Chemical Society . 134 (42): 17482–17485. doi:10.1021/ja308826x. PMID 23039372.