Acetone oxime
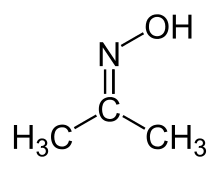
Acetone oxime (acetoxime) is the organic compound with the formula (CH3)2CNOH. It is the simplest example of a ketoxime. It is a white crystalline solid that is soluble in water, ethanol, ether, chloroform, and ligroin. It is used as a reagent in organic synthesis.[2]
 | |
| Names | |
|---|---|
| IUPAC name
2-Propanone oxime | |
| Other names
Acetoxime; N-Hydroxy-2-propanimine; Methyl methyl ketoxime | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.004.383 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H7NO | |
| Molar mass | 73.095 g·mol−1 |
| Appearance | White needle like crystals |
| Density | 0.901 g/mL[1] |
| Melting point | 60 to 63 °C (140 to 145 °F; 333 to 336 K) |
| Boiling point | 135 °C (275 °F; 408 K) |
| 330 g/L (20 °C) | |
| -44.42·10−6 cm3/mol | |
| Hazards | |
| Main hazards | May be harmful if swallowed |
| GHS pictograms |     |
| GHS Signal word | Danger |
| H228, H302, H317, H318, H351 | |
| P201, P202, P210, P240, P241, P261, P264, P270, P272, P280, P281, P301+312, P302+352, P305+351+338, P308+313, P310, P321, P330, P333+313, P363, P370+378, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 60 °C (140 °F; 333 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
4,000 mg/kg Intraperitoneal-mouse |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Acetone oxime (acetoxime) was first prepared and named in 1882 by the German chemist Victor Meyer and his Swiss student Alois Janny.[3]
Preparation
Acetone oxime is synthesized by the condensation of acetone and hydroxylamine in the presence of HCl:[4][2]
- (CH3)2CO + H2NOH → (CH3)2CNOH + H2O
It can also be generated via ammoxidation of acetone in the presence of hydrogen peroxide.[5]
Uses
Acetone oxime is an excellent corrosion inhibitor (deoxidant) with lower toxicity and greater stability compared to the common agent hydrazine. It is also useful in the determination of ketones, cobalt and in organic synthesis.[6]
References
- Sigma-Aldrich Chemical Catalogue "Acetone Oxime". Retrieved 2 September 2016.
- Steven M. Weinreb, Kristina Borstnik "Acetone Oxime" e-EROS Encyclopedia of Reagents for Organic Synthesis, 2007. doi:10.1002/047084289X.rn00765
- Meyer, Victor; Janny, Alois (1882). "Ueber die Einwirkung von Hydroxylamin auf Aceton" [On the effect of hydroxylamine on acetone]. Berichte der Deutschen Chemischen Gesellschaft (in German). 15: 1324–1326. doi:10.1002/cber.188201501285. From p. 1324: "Die Substanz, welche wir, wegen ihrer nahen Beziehungen zur Acetoximsäure, und da sie keine sauren Eigenschaften besitzt, vorläufig Acetoxim nennen wollen, …" (The substance, which we – on account of its close relations to acetoximic acid, and since it possesses no acid properties – will, for the present, name "acetoxime," … )
- Handbook of Chemistry and Physics "Acetone Oxime". Retrieved 23 April 2014.
- Xinhua Liang, Zhentao Mi, Yaquan Wang, Li Wang, Xiangwen Zhang "Synthesis of acetone oxime through acetone ammoximation over TS-1" Reaction Kinetics and Catalysis Letters Volume 82, pp 333-337. .
- Acetone Oxime Properties, additional text.
