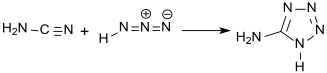5-Aminotetrazole
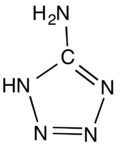
5-Aminotetrazole is an organic compound with the formula HN4CNH2. It is a white solid that can be obtained both in anhydrous and hydrated forms.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
1H-Tetrazol-5-ylamine | |
| Other names
5-ATZ | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.022.348 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| CH3N5 | |
| Molar mass | 85.070 g·mol−1 |
| Appearance | White solid |
| Density | 1.502 g/cm3 |
| Melting point | 201–205 °C (394–401 °F; 474–478 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The molecule is planar.[1] The hydrogen bonding pattern in the hydrate supports the assignment of NH being adjacent to carbon in the ring.[2]
Preparation
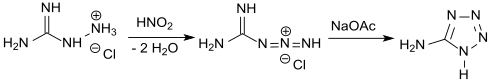
A synthesis of 5-aminotetrazole through the action of nitrous acid on aminoguanidine was reported by Johannes Thiele in 1892.[3] The exact structure of the compound was not known at the time, although it was known to crystallize as a monohydrate when prepared in aqueous solution.
The correct structural formula was published in 1901 by Arthur Hantzsch, who obtained it from the reaction between cyanamide and hydrazoic acid.[4]
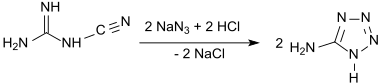
To avoid direct handling of the problematic hydrazoic acid, a mixture of sodium azide and hydrochloric acid has been used and gave the monohydrate at 73% yield.[5]
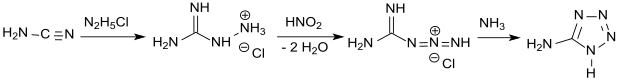
In a much more efficient and controllable one-pot synthesis, cyanamide is reacted with hydrazine hydrochloride to give aminoguanidine hydrochloride, which is then diazotized as in Thiele's original process. Addition of ammonia or sodium hydroxide to decrease the acidity followed by heat-induced cyclization gives the anhydrous product in 74% yield.[6][7]
Uses
5-Aminotetrazole has found applications in heterocyclic chemistry, particularly as a synthon for numerous multicomponent reactions.[8]
The compound has a particularly high nitrogen content of 80%. Partly for this reason, the compound is prone to decomposition to nitrogen gas (N2). It has been widely investigated for gas-generating systems, such as airbags and blowing agents.[9]
References
- Hiroshi Fujihisa, Kazumasa Honda, Shigeaki Obata, Hiroshi Yamawaki, Satoshi Takeya, Yoshito Gotoha, Takehiro Matsunaga "Crystal structure of anhydrous 5-aminotetrazole and its high-pressure behavior" CrystEngComm, 2011, volume 13, pp. 99-102. doi:10.1039/C0CE00278J
- D. D. Bray and J. G. White "Refinement of the structure of 5-aminotetrazole monohydrate" Acta Crystallogr. (1979). B35, pp. 3089-3091.doi:10.1107/S0567740879011493
- Thiele, Johannes (1892-01-01). "Ueber Nitro- und Amidoguanidin". Justus Liebigs Annalen der Chemie. 270 (1‐2): 1–63. doi:10.1002/jlac.18922700102. ISSN 0075-4617.
- Hantzsch, A.; Vagt, A. (1901-01-01). "Ueber das sogenannte Diazoguanidin". Justus Liebigs Annalen der Chemie. 314 (3): 339–369. doi:10.1002/jlac.19013140307. ISSN 0075-4617.
- MIHINA, JOSEPH S.; HERBST, ROBERT M. (1950-09-01). "The Reaction of Nitriles with Hydrazoic Acid: Synthesis of Monosubstituted Tetrazoles". The Journal of Organic Chemistry. 15 (5): 1082–1092. doi:10.1021/jo01151a027. ISSN 0022-3263.
- US 5424449, Rothgery, Eugene F. & Karl O. Knollmueller, "Process for the preparation of 5-aminotetrazole", published 1995-06-13, issued 1995-06-13
- US 5594146, Murotani, Masahiro; Hajime Mura & Makoto Takeda et al., "Process for producing 5-aminotetrazole", published 1997-01-14, issued 1997-01-14
- Dolzhenko, A. V. (2017). "5-Aminotetrazole as a Building Block for Multicomponent Reactions (Review)". Heterocycles. 94 (10): 1819–1846. doi:10.3987/rev-17-867.
- Lesnikovich, A. I.; Ivashkevich, O. A.; Levchik, S. V.; Balabanovich, A. I.; Gaponik, P. N.; Kulak, A. A. "Thermal decomposition of aminotetrazoles" Thermochimica Acta 2002, vol. 388, pp. 233-251. doi:10.1016/S0040-6031(02)00027-8