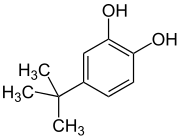
4-tert-Butylcatechol
4-tert-Butylcatechol (TBC) is an organic chemical compound which is a derivative of catechol.[1] TBC is available in the form of a solid crystal flake[2] and 85% solution in methanol[3] or water.[4]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
4-tert-Butylbenzene-1,2-diol | |
| Other names
para-tert-Butylcatechol p-tert-Butylcatechol t-Butyl catechol p-t-Butylpyrocatechol p-tert-Butylpyrocatechol 4-t-Butylpyrocatechol 4-tert-Butylpyrocatechol | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | TBC |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.413 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H14O2 | |
| Molar mass | 166.217 g/mol |
| Melting point | 50 °C |
| Boiling point | 285 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Uses
It is added as a stabilizer and polymerisation inhibitor to butadiene, styrene,[5] vinyl acetate, divinylbenzene[6] and other reactive monomer streams.[7]
TBC is also used as a stabilizer in the manufacture of polyurethane foam.[8] It also can be used as an antioxidant for synthetic rubber, polymers and oil derivatives.[7] It can be used as purification agent for aminoformate catalysts.
It is 25 times better than hydroquinone at 60 °C for polymerization inhibitory effect.
See also
References
- "Nomination Background: p-tert butylcatechol" (PDF). Nomination Background: p-tert butylcatechol. April 1993. Retrieved 4 September 2020.
- "TBC OPTIMA 100% FLAKES". Solvay. Retrieved 2020-09-04.
- "TBC OPTIMA 85% METHANOL". Solvay. Retrieved 2020-09-04.
- "TBC OPTIMA 85% WATER". Solvay. Retrieved 2020-09-04.
- "Styrene Safe Handling and Storage Guide" (PDF). Americas Styrenics. Retrieved 2020-09-04.
- "DuPont Divinylbenzene (TM) Technical Manual" (PDF). 2020-03-01. Retrieved 2020-09-04.
- "4-Tertiary Butylcatechol | Business & Products". DIC Corporation. Retrieved 2020-09-04.
- "tert-Butylcatechol (TBC)". Silver Fern Chemical Inc. Retrieved 2020-09-04.
Sources
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.