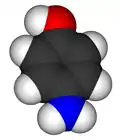
4-Aminophenol
4-Aminophenol (or para-aminophenol or p-aminophenol) is the organic compound with the formula H2NC6H4OH. Typically available as a white powder,[3] it was commonly used as a developer for black-and-white film, marketed under the name Rodinal.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
4-Aminophenol[1] | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol) |
|||
| 385836 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.198 | ||
| EC Number |
| ||
| 2926 | |||
| KEGG | |||
| MeSH | Aminophenols | ||
PubChem CID |
|||
| UNII | |||
| UN number | 2512 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C6H7NO | |||
| Molar mass | 109.128 g·mol−1 | ||
| Appearance | Colorless to reddish-yellow crystals | ||
| Density | 1.13 g/cm3 | ||
| Melting point | 187.5 °C (369.5 °F; 460.6 K) | ||
| Boiling point | 284 °C (543 °F; 557 K) | ||
| 1.5 g/100 mL | |||
| Solubility |
| ||
| log P | 0.04 | ||
| Acidity (pKa) |
| ||
| Structure | |||
| orthorhombic | |||
| Thermochemistry | |||
Std enthalpy of formation (ΔfH⦵298) |
-190.6 kJ/mol | ||
| Hazards | |||
| GHS pictograms |    | ||
| GHS Signal word | Warning | ||
| H302, H332, H341, H400, H410 | |||
| P201, P202, P261, P264, P270, P271, P273, P281, P301+312, P304+312, P304+340, P308+313, P312, P330, P391, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 195 °C (383 °F; 468 K) (cc) | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
671 mg/kg | ||
| Related compounds | |||
Related aminophenols |
2-Aminophenol 3-Aminophenol | ||
Related compounds |
Aniline Phenol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Reflecting its slightly hydrophilic character, the white powder is moderately soluble in alcohols and can be recrystallized from hot water. In the presence of a base, it oxidizes readily. The methylated derivatives N-methylaminophenol and N,N-dimethylaminophenol are of commercial value.
The compound is one of three isomeric aminophenols, the other two being 2-aminophenol and 3-aminophenol.
Preparation
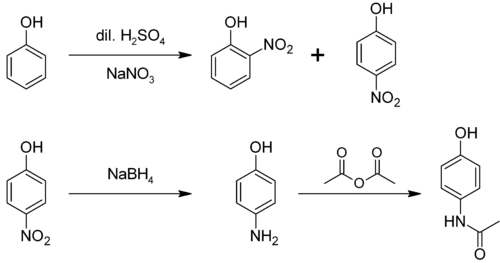
From phenol
It is produced from phenol by nitration followed by reduction with iron. Alternatively, the partial hydrogenation of nitrobenzene affords phenylhydroxylamine, which rearranges primarily to 4-aminophenol:[4]
- C6H5NO2 + 2 H2 → C6H5NHOH + H2O
- C6H5NHOH → HOC6H4NH2
From nitrobenzene
It can be produced from nitrobenzene by electrolytic conversion to phenylhydroxylamine, which spontaneously rearranges to 4-aminophenol.[5]
Uses
4-Aminophenol is a building block used in organic chemistry. Prominently, it is the final intermediate in the industrial synthesis of paracetamol. Treating 4-aminophenol with acetic anhydride gives paracetamol:[6][7][8]
References
- Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 690. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. pp. 5–89. ISBN 978-1498754286.
- CRC Handbook of Chemistry and Physics 65th Ed.
- Mitchell, S.C. & Waring, R.H. "Aminophenols." In Ullmann’s Encyclopedia of Industrial Chemistry; 2002 Wiley-VCH, doi:10.1002/14356007.a02_099
- Polat, K.; Aksu, M.L.; Pekel, A.T. (2002), "Electroreduction of nitrobenzene to p-aminophenol using voltammetric and semipilot scale preparative electrolysis techniques", Journal of Applied Electrochemistry, Kluwer Academic Publishers, 32: 217–223, doi:10.1023/A:1014725116051
- Ellis, Frank (2002). Paracetamol: a curriculum resource. Cambridge: Royal Society of Chemistry. ISBN 0-85404-375-6.
- Anthony S. Travis (2007). "Manufacture and uses of the anilines: A vast array of processes and products". In Zvi Rappoport (ed.). The chemistry of Anilines Part 1. Wiley. p. 764. ISBN 978-0-470-87171-3.
- Elmar Friderichs; Thomas Christoph; Helmut Buschmann. "Analgesics and Antipyretics". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_269.pub2.