4-Amino-3-hydrazino-5-mercapto-1,2,4-triazole
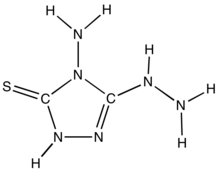
4-Amino-3-hydrazino-5-mercapto-1,2,4-triazole is an organic compound with the formula SC2N3H(NH2)(N2H3). The compound consists of a 1,2,4-triazole heterocycle with three functional groups: amine, thioamide, hydrazide. X-ray crystallography shows that this molecule is polar but with a C=S double bond. It is prepared by the reaction of hydrazine with thiourea:[1]
- 2 SC(NH2)2 + 3 N2H4 → SC2N3H(NH2)(N2H3) + 4 NH3 + H2S
 | |
| Names | |
|---|---|
| Other names
Purpald; AHMT; 1,2,4-Triazolidin-3-one, 4-amino-5-thioxo-, hydrazone (9CI); 4H-1,2,4-Triazole-3-thiol, 4-amino-5-hydrazino- (6CI,7CI) | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.015.578 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2H6N6S | |
| Molar mass | 146.17 |
| Appearance | white solid |
| Density | 1.69 g/cm3 |
| Melting point | 228 °C (442 °F; 501 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The compound has been used as a reagent for the colorimetric detection of aldehydes.[2]
References
- N. W. Isaacs and C. H. L. Kennard "Crystal structure of 4-amino-3-hydrazino-5-mercapto-1,2,4-triazole" J. Chem. Soc. B, 1971, 1270-1273. doi:10.1039/J29710001270
- Hopps, Harvey B. "Purpald: a Reagent that Turns Aldehydes Purple!" Aldrichimica Acta 2000, volume 33, pp. 28-30.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.