3-Methylornithine
3-Methylornithine is an amino acid with the formula H2N(CH2)2CH(CH3)CH(NH2)CO2H. This amino acid contains two stereogenic centers, but only one stereoisomer (namely (3R)-3-methyl-D-ornithine) occurs in nature. It is produced from lysine by the action of the enzyme methylornithine synthase. The combination of lysine and 3-methylornithine, also mediated enzymatically, produces pyrrolysine, which, for a few organisms, is a "22nd" genetically coded amino acid.[1][2]

Structure of pyrrolysine, which is biosynthesized from methylornithine and lysine
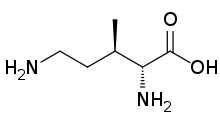 | |
| Names | |
|---|---|
| Other names
5-Aminoisoleucine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| |
| |
| Properties | |
| C6H14N2O2 | |
| Molar mass | 146.190 g·mol−1 |
| Appearance | White solid |
| Density | 1.121 g/cm3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- Gaston, Marsha A.; Jiang, Ruisheng; Krzycki, Joseph A. "Functional Context, Biosynthesis, and Genetic Encoding of Pyrrolysine" Current Opinion in Microbiology 2011, vol. 14, pp. 342-349. doi:10.1016/j.mib.2011.04.001
- Quitterer, F.; Beck, P.; Bacher, A.; Groll, M., "Structure and Reaction Mechanism of Pyrrolysine Synthase (PylD)", Angew. Chem. Int. Ed. 2013, volume 52, pp. 7033-7037. doi:10.1002/anie.201301164
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.